The spin-only magnetic moment of [CrF6]4 ( atomic number of Cr is 24) is
A
0
B
1.73 BM
C
2.83 BM
D
4.9 BM
Video Solution
Text Solution
The correct Answer is:D
.24Cr=[Ar]3d54s1,Cr2+
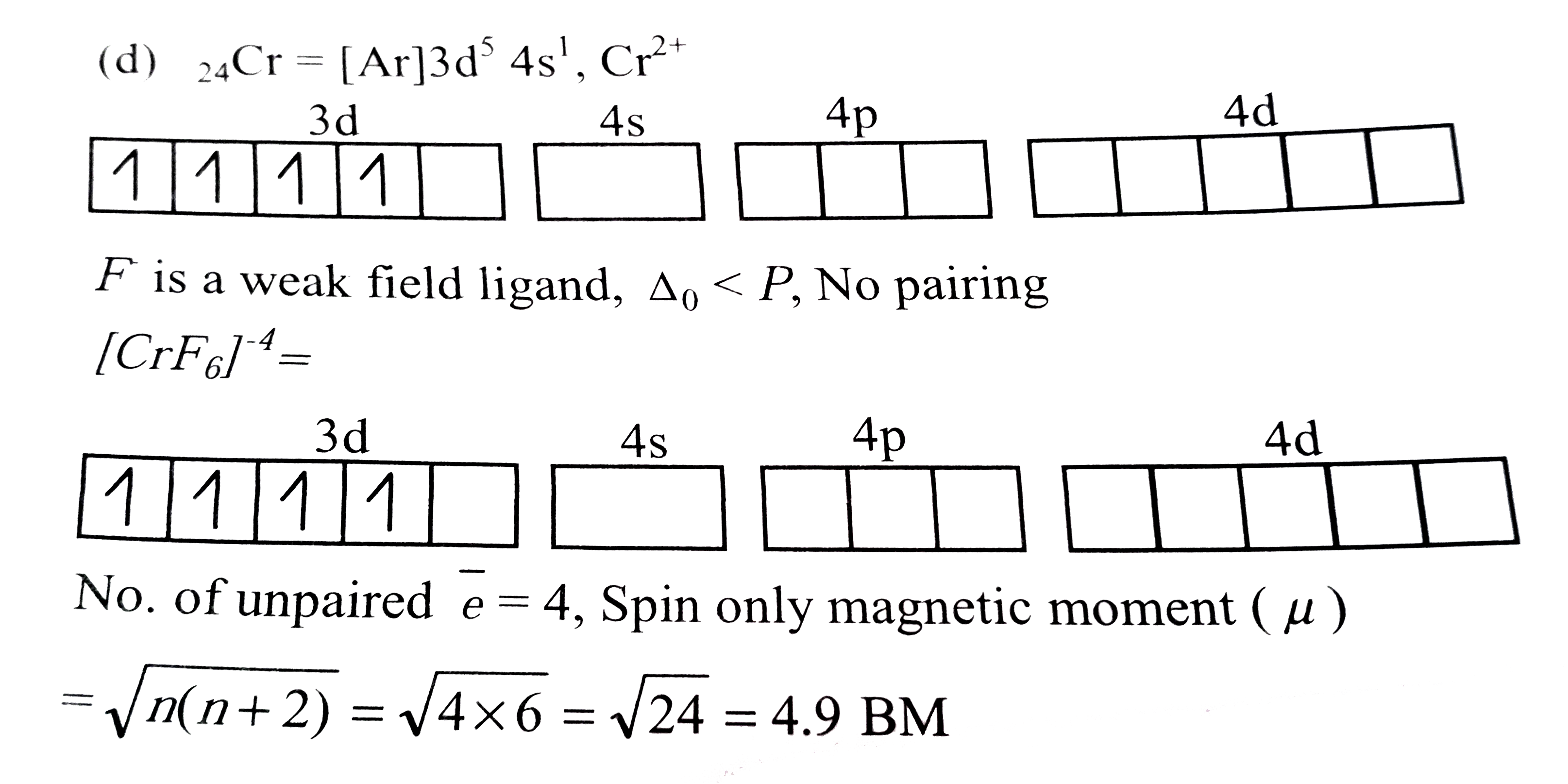
No. of unpaired bar€ = 4, Spin only magnetic moment (μ)
=√n(n+2)=√4×6=√24=4.9BM
|
Similar Questions
Knowledge Check
The spin only magnetic moment of Mn4+ ion is nearly
A3BMB6BMC4BMD5BMThe spin only magnetic moment of Mn4+ ion is nearly
A3 BMB6 BMC4 BMD5 BMThe calculated spin only magnetic moment of Cr2+ ion is
A3.87 BMB4.90 BMC5.92 BMD2.84 BM
The calculated spin only magnetic moment of Cr2+ ion is:
View SolutionThe calculated spin only magnetic moment of Cr2+ ion is
View SolutionThe calculated spin only magnetic moment of Cr2+ ion is
View SolutionThe spin only magnetic moment of Mn4+ ion is nearly
View SolutionThe spin-only magnetic moments of [Mn(CN)6]4− and [MnBr4]2− in Bohr Magnetons, respectively, are
View Solution
