Which of the following complex will give white ppt. with BaCl2 solution ?
A
[Co(NH3)4SO4]NO2
B
[Cr(NH3)5SO4]Cl
C
[Cr(NH3)5Cl]SO4
D
Both (b) and (c )
Video Solution
Text Solution
The correct Answer is:C
[Cr(NH3)5Cl]SO4→[Cr(NH3)5Cl]2++SO2−4
[Cr(NH3)5Cl]2++SO2−4+BaCl2→[Cr(NH3)5Cl]Cl2+BaSO4+HCl
|
Similar Questions
Knowledge Check
Which of the following isomers will give white precipitate with BaCl2 solution ?
A[Co(NH3)5SO4]BrB[Co(NH3)5Br]SO4C[Co(NH3)4(SO4)2]BrD[Co(NH3)4Br(SO4)]Which of the following comolexes wll give white precipiltate with BaCI2 solution ?
A[Cr(NH3)5SO4]CIB[Co(NH3)4CI2[NO2C[Cr(NH3)5CI]SO4DBoth (a) and (c)Which of the following will give white ppt. with aq.AgNO3 ?
APh-ClB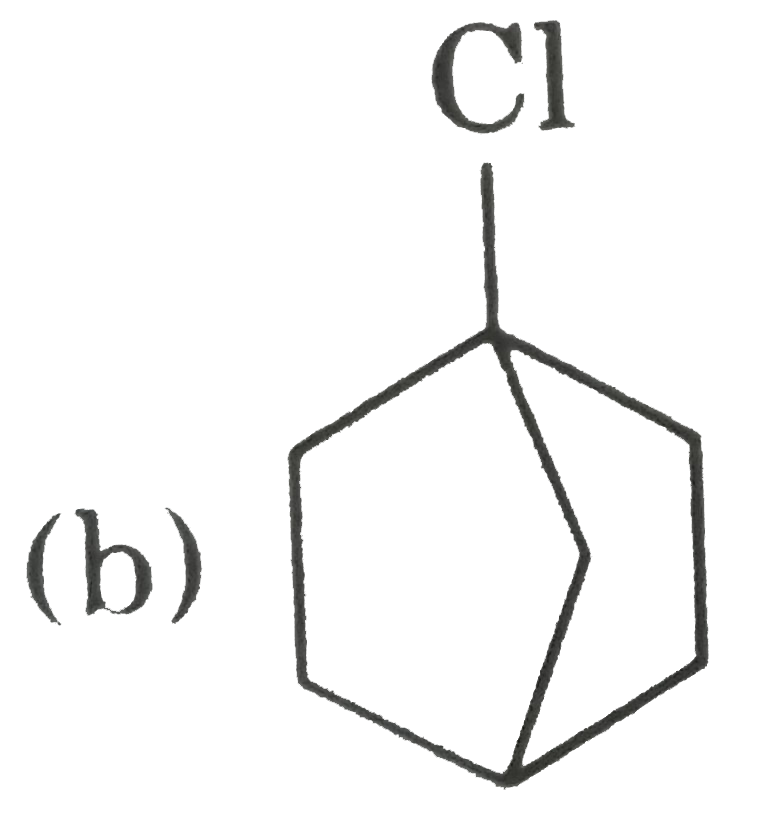 CPh−CH2−IDCH3−CH2CH2CH2CH2−Cl
CPh−CH2−IDCH3−CH2CH2CH2CH2−Cl
Which of the following compound are having white ppt ?
View SolutionWhich of the following complex will give white precipitate with barium chloride solution –
View SolutionWhich of the followings compounds will gives white ppt. with ammonical silver nitrate solution (Tollen's reagment)?
View SolutionWhich of the following complex will give a white precipitate on treatment with BaCl2 solution ?
View SolutionWhich of the following complex will give a white precipitate on treatment with BaCl2.solution ?
View Solution

