The number of unpaired electrons calculated in [Co(NH3)6]3+ and |CoF6|3− are
A
4 and 4
B
0 and 2
C
2 and 4
D
0 and 4
Video Solution
Text Solution
The correct Answer is:D
The formationof complex compound depends upon the nature of the ligand whether they are strong or weak.
The number of unpaired electrons −[Co(NH3)6]3+→ Inner orbital complex ,d2sp3 hybridisation
Co3→1s22s22p63s23p64s03d6
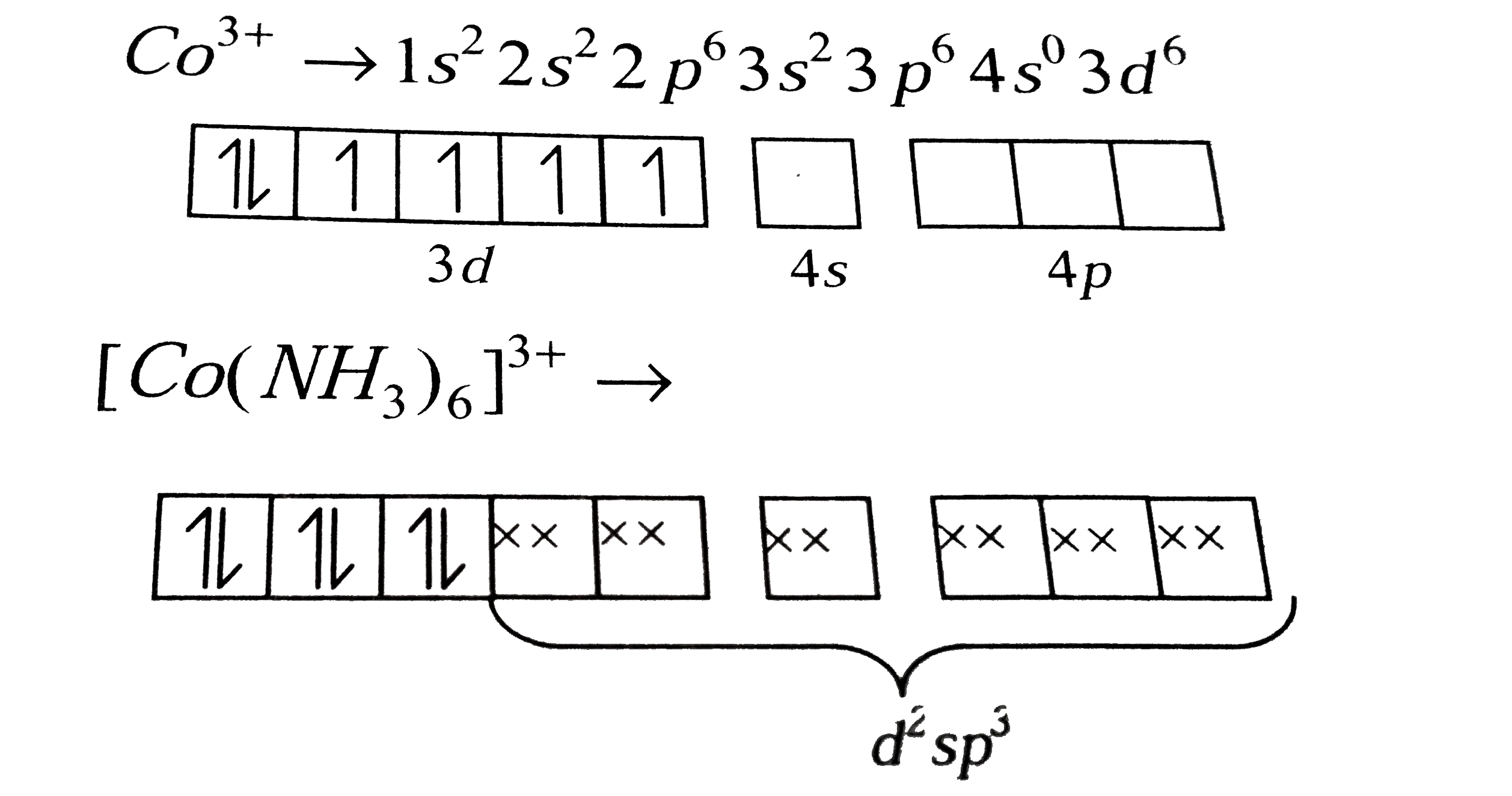
unpaired electron = zero (diamagnetic )
[CoF6]3−→ Outer orbital complex, sp3d2 hybridisation
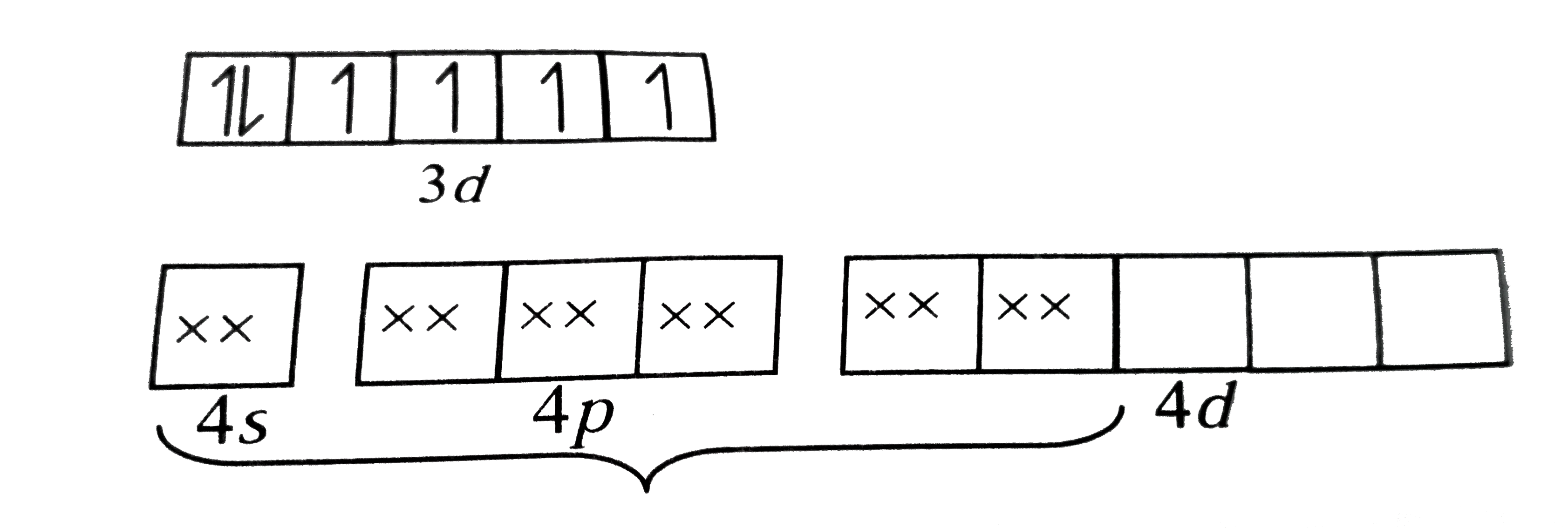
Unpaired electrons =four (paramagnetic)
|
Similar Questions
Knowledge Check
The number of unpaired electrons calsulated in [Co(NH3)3]3+ and |CoF6|3− are
A4 and 4B0 and 2C2 and 4D0 and 4Number of unpaired electrons present in[Ni(H2O)6]3+
ATwoBOneCFourDThree[CoF6]3− is
Aparamagnetic and under sp3d2 hybridisationBdiamagnetic and undergoes d2sp3 hybridisationCparagamagnetic and undergoes sp3d hybridisationDdiamagnetic and undergoes sp3 hybridisation.
The total number of unpaired electrons present in [Co(NH3)6]Cl2and[Co(NH3)6]Cl3 is
View SolutionNumber of unpaired electrons in [Mn(CN)6]3− is
View SolutionNumber of unpaired electrons in [Mn(CN)_(6)]^(3-) is
View SolutionThe number of unpaired electrons in the complex ion [CoF6]3− is
View SolutionThe number of unpaired electrons expected for the complex ion [Cr(NH3)6]2+ is :
View Solution

