Octahedral complex shows which form of geometrical isomerism 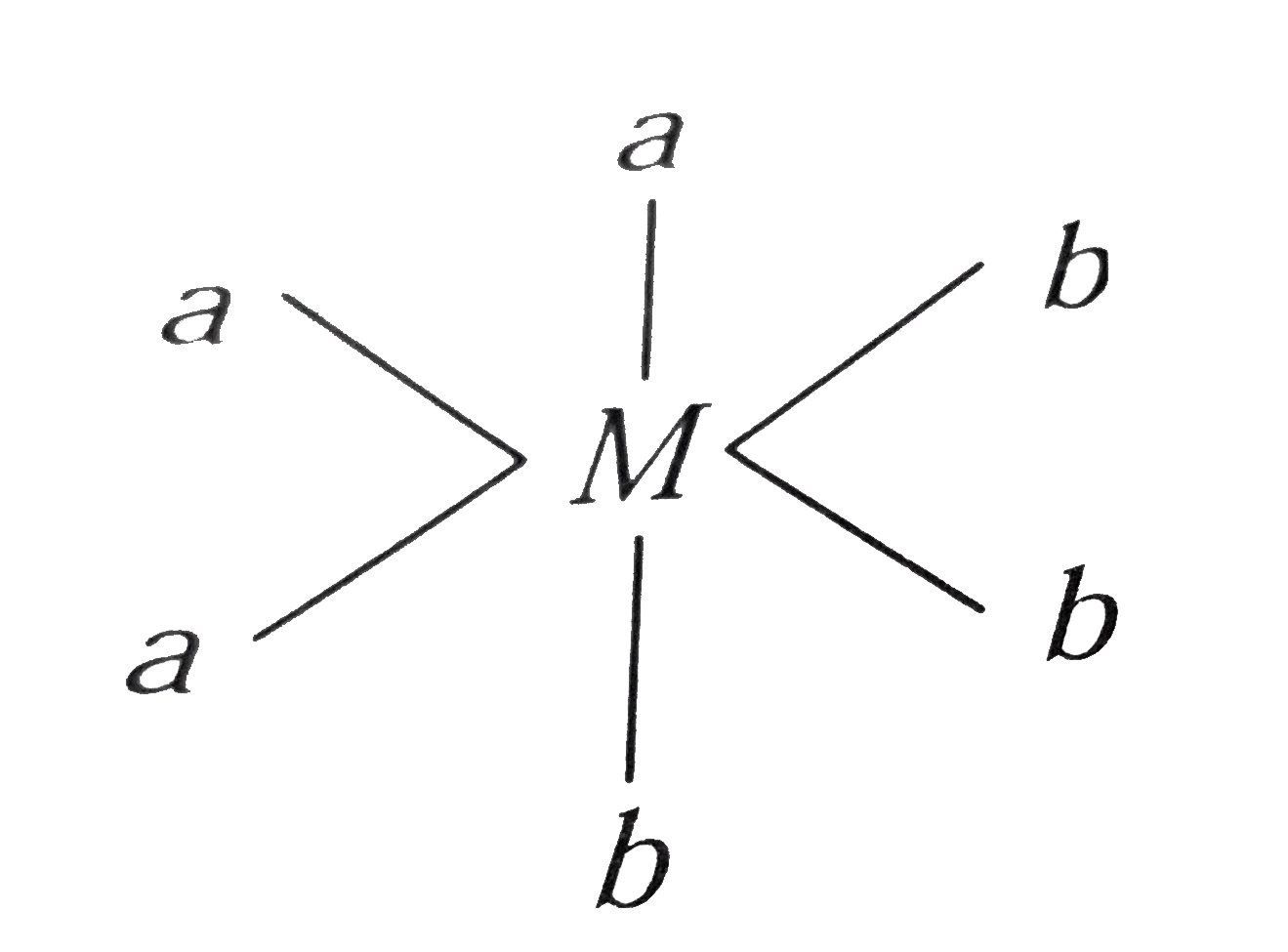
A
cis
B
trans
C
mer
D
fac
Video Solution
Text Solution
The correct Answer is:D
When the three ligands ( with same donor atoms ) are on the same triangular face of the octahedron, the isomer is called facial or fac isomer.
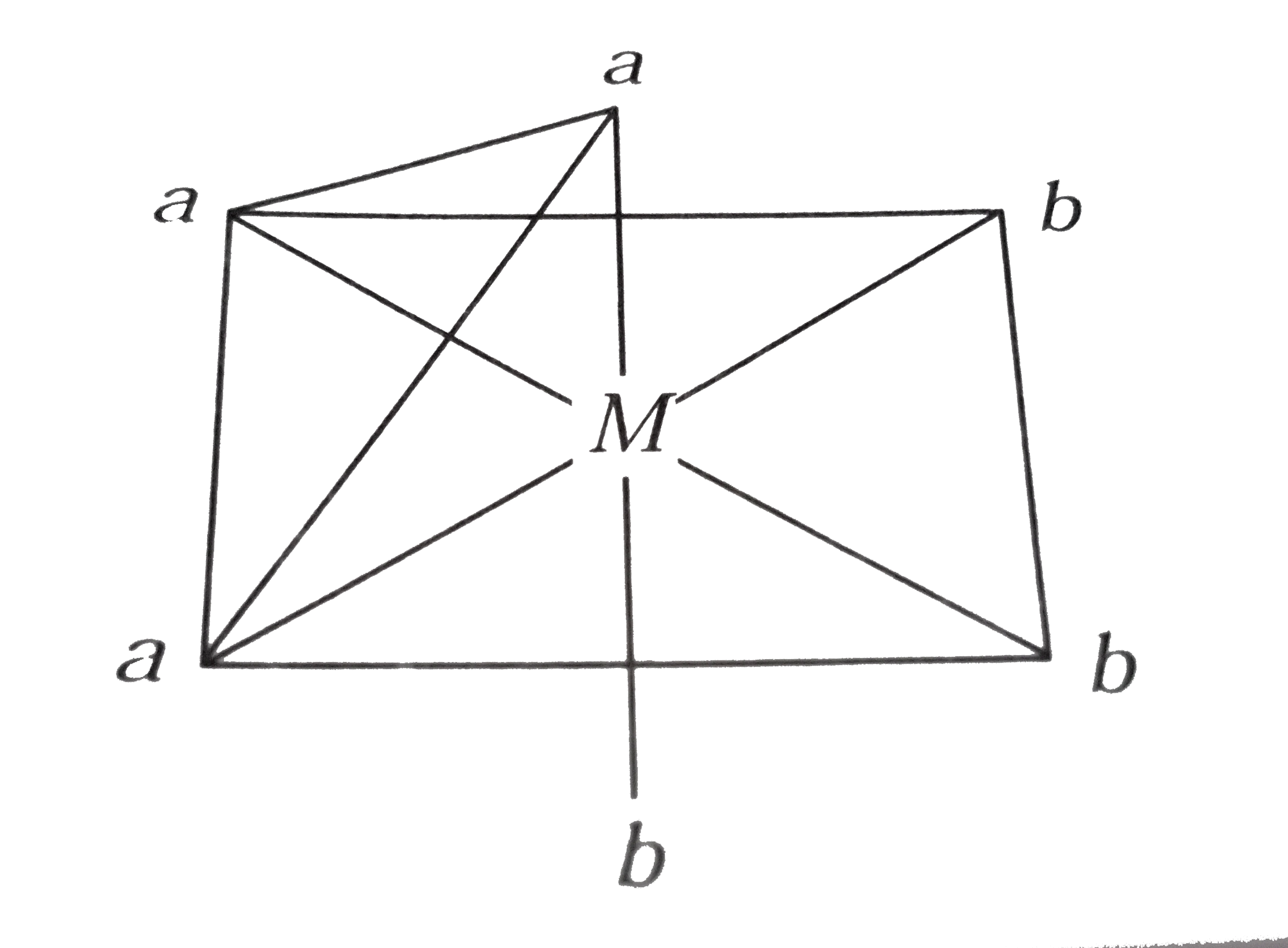
In this complex the three ligands are on the same triangular face of the octahedral.
|
Similar Questions
Knowledge Check
Which one of following octahedral complexes will not show geometrical isomerism ?
AM[A2B2]BM[A3B3]C[MA4B2]D[MA5B]Which show geometrical isomerism?
A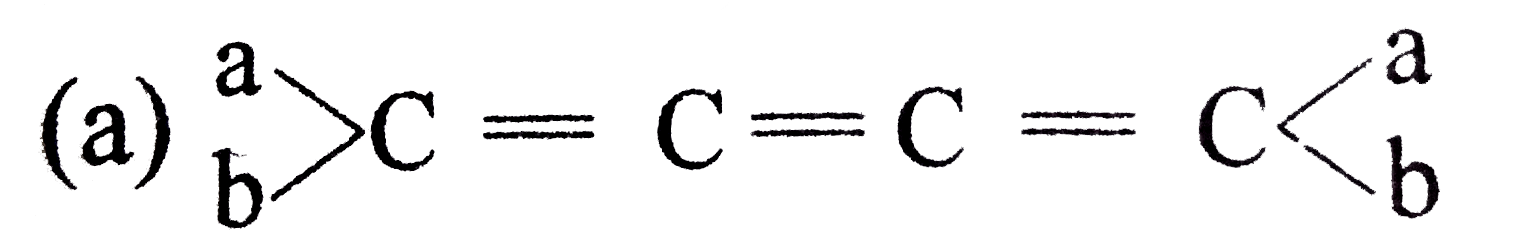 B
B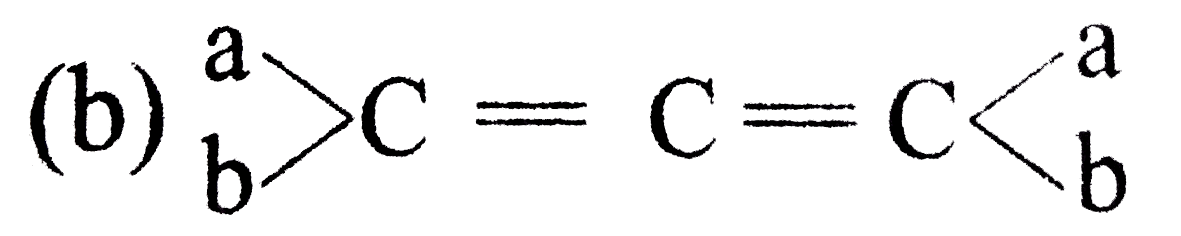 C
C DNone of these
DNone of theseWhich of the following octahedral complexes do not show geometrical isomerism ?
A[Co(NH3)3Cl3]B[PtCl2(NH3)4]C[Cr(H2O)5Cl]2+D[Co(en)3]3+
Which will show geometrical isomerism ?
View SolutionWhich will show geometrical isomerism ?
View SolutionWhich of the following octahedral complexes do not show geometrical isomerism?
View SolutionWhich one of the following octahedral complexes will not show geometrical isomerism ? (A and B are monodentate ligands)
View SolutionWhich one of the following octahedral complexes will not show geometrical isomerism ? (A and B are monodentate ligands)
View Solution