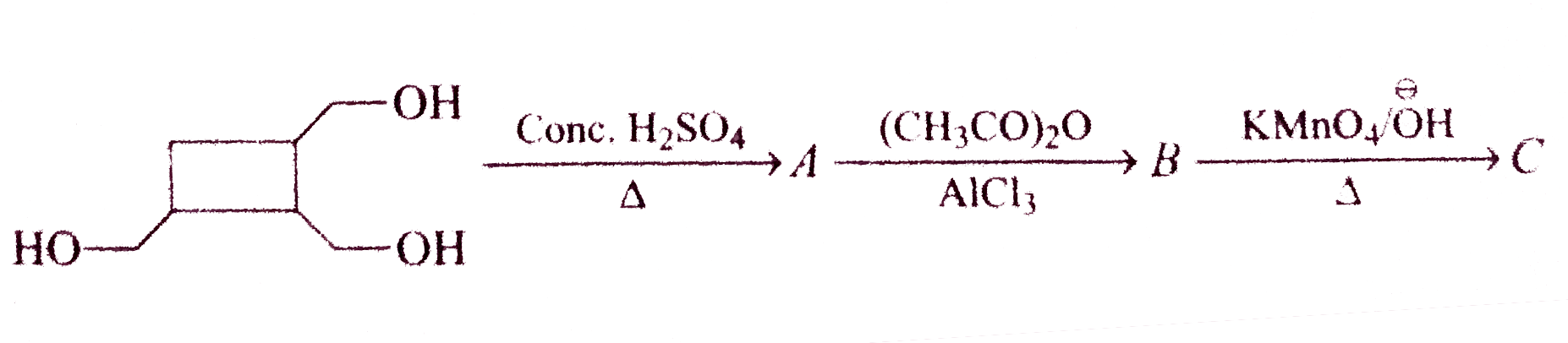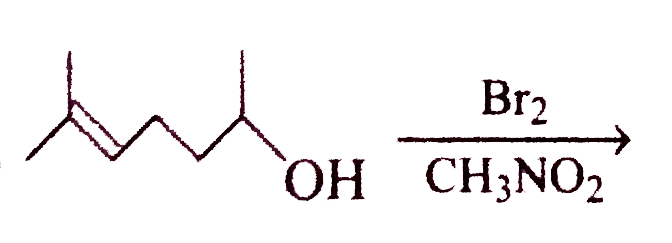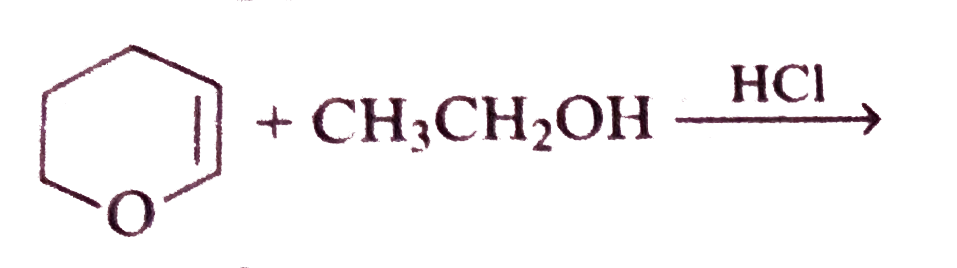The final product of complete oxidation of hydrocarbons is
A
Acid
B
Aldehyde
C
H2O+CO2
D
Dihydric alcohol
Video Solution
Text Solution
The correct Answer is:C
CH4Methane+2O2→CO2+2H2O
C2H4Ethene+3O2→2CO2+2H2O
All hydrocarbons saturated or unsaturated on complete combustion always produce CO2 and H2O
|
Similar Questions
Knowledge Check
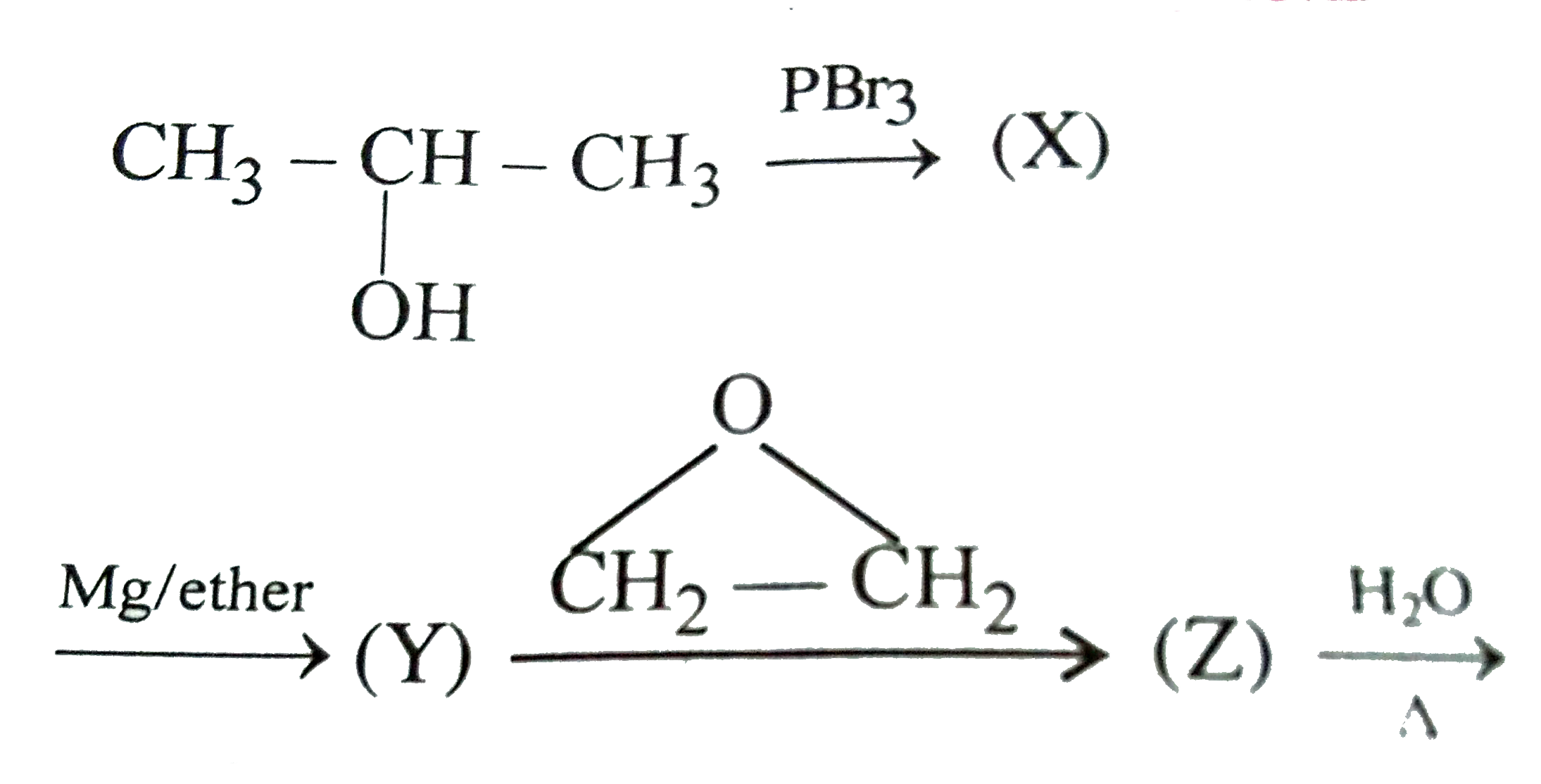
The final product isACH−CH∣CH3−CH2CH2OHBCH3−O−CH∣CH3−CH2−CH3CCH3−CH∣CH3−O−CH2−CH3DNone of the above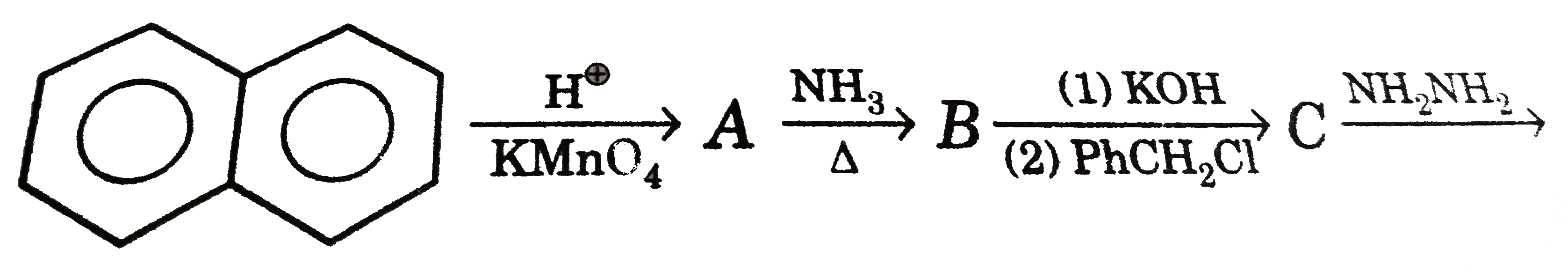
final product isAPhNH2BPhNHNH2C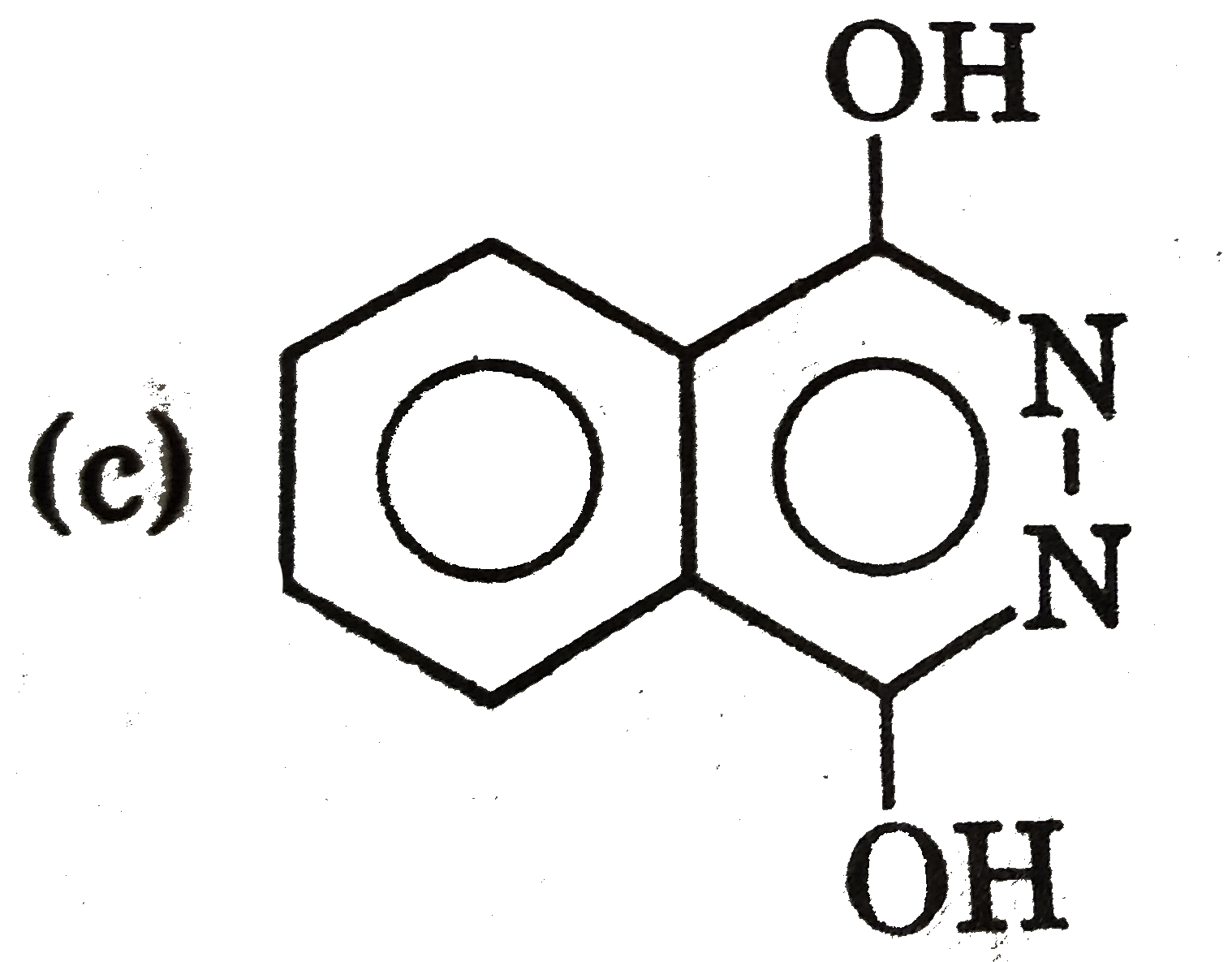 DPhCH2NH2
DPhCH2NH2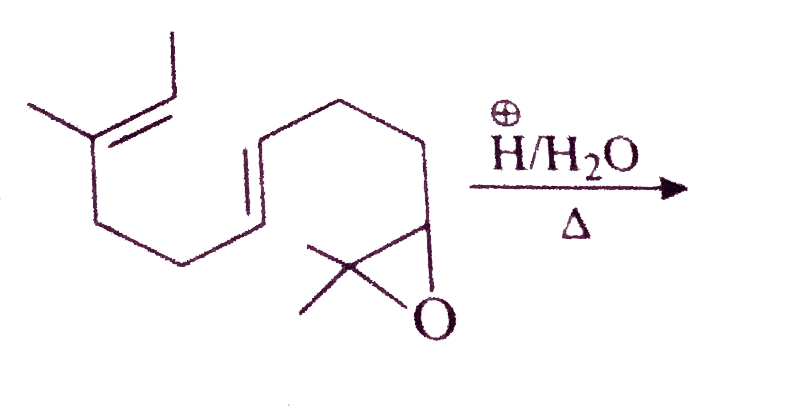
The final product is :A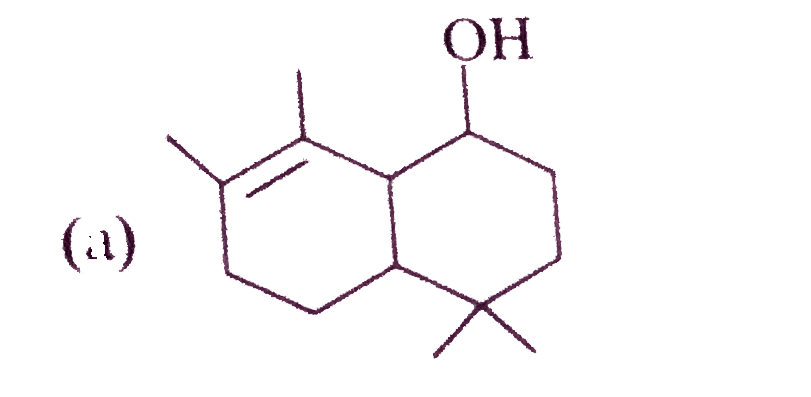 B
B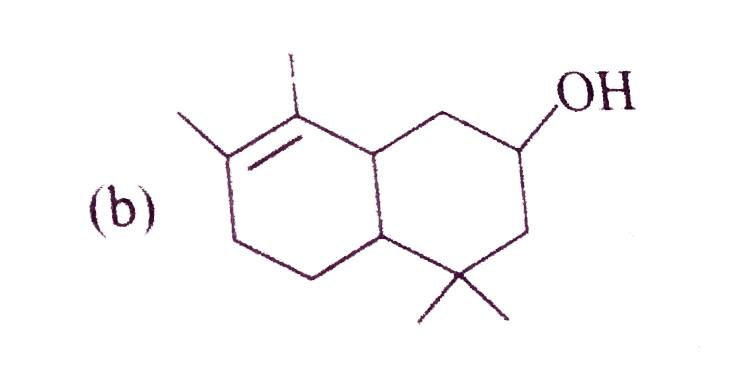 C
C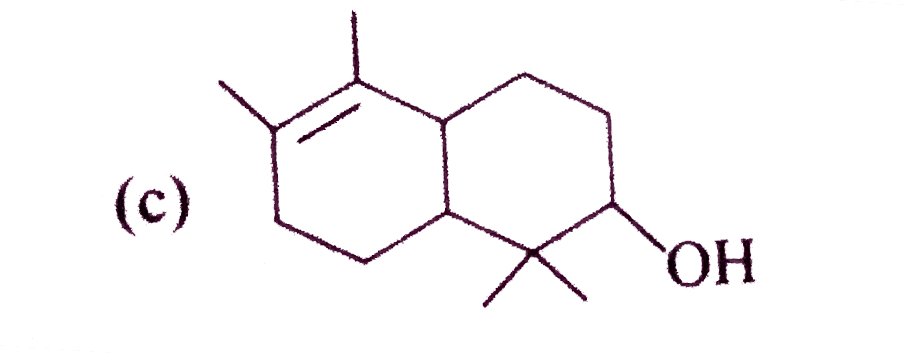 D
D