In paraffins, with the increasing molecular weight, it is found that
A
Freezing point decreases
B
Boiling point decreases
C
Boiling point increases
D
Vapour pressure decreases
Video Solution
Text Solution
The correct Answer is:C
Paraffins are non-polar compounds. The intermolecular forces are weak Vander Waal's forces. As the molecular mass increases Vander Waal's forces increases. Hence boiling point increases.
|
Similar Questions
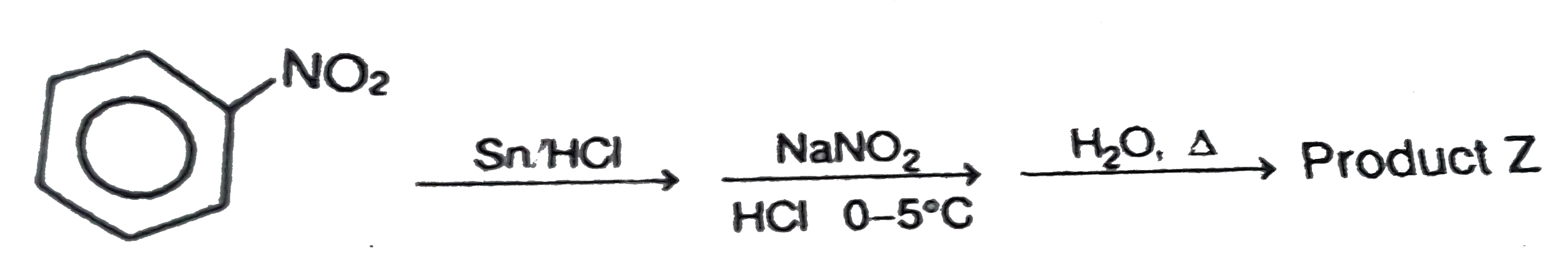
Find the molecular weight of Z.
View SolutionStatement I: The solubility of n-alcohol in water decrease with an increase in molecular weight.
Statement II: The reactive proportion of the hydrocarbon part in alcohols increase with increasing molecular weight which permits enthanced hydrogen bonding with water.
View Solution
Knowledge Check
With the increasesing molecular weight of a liquid the viscosity
AdecreasesBincreasesCno effectDall are wrongThe boiling points of halogens increase with increase in molecular weight, it is because
AAs the size increases molecules undergo association leading to higher stabilityBBond strength increases due to increase in electronegativityCvan der Waals force increase with increase in number of electrons per moleDNone of the aboveStatement-I : Solubility on n-alcohols in water decreases with increase in molecular weight
Because
Statement-II: The relative proportion of the hydrocarbon part in alcohols increases with increasing molecular weight which permits enhanced hydrogen bond with water.AStatement-I is True, Statement-II is True : Statement-II is a correct explanation for Statement-IBStatement-I is True, Statement-II is True : Statement-II is NOT a correct explanation for Statement-ICStatement-I is True, Statement-II is False.DStatement-I is False, Statement-II is True.
Paraffins are
View SolutionStatement I Solubility of n-alcohol in water decreases with increase in molecular weight.
Statement II The relative proportion of the hydrocarbon part in alcohols increases with increasing molecular weight which permit enhanced hydrogen with water.
View SolutionParaffin wax is
View SolutionParaffin wax is a/ an _______.
View SolutionParaffin wax is
View Solution

