CH3−C≡CHO3−−−−−→Zn/H2O2 Product . Product in above reaction is
CH3COOH
HCOOH
Both (a) and (b)
CH3CHO+HCHO
The correct Answer is:C
To solve the question, we need to analyze the reaction involving the compound CH3−C≡CH (which is propyne) undergoing ozonolysis followed by treatment with zinc and hydrogen peroxide.
1. Identify the Compound: The starting compound is propyne, which can be represented as CH3−C≡CH.
2. Ozonolysis Reaction: Ozonolysis is a reaction where an alkene or alkyne is treated with ozone (O3) to form ozonides, which can then be further processed to yield carbonyl compounds (aldehydes or ketones). In this case, propyne will undergo ozonolysis.
3. Formation of Ozonide: When propyne reacts with ozone, it forms an ozonide intermediate. The structure of the ozonide will depend on the cleavage of the triple bond.
4. Reduction of Ozonide: The ozonide is then treated with zinc (Zn) and hydrogen peroxide (H2O2). This step is crucial as it leads to oxidative cleavage of the ozonide.
5. Products of the Reaction: The oxidative ozonolysis of propyne will yield two products:
- Acetic acid (CH3COOH)
- Formic acid (HCOOH)
6. Conclusion: Therefore, the final products of the reaction CH3−C≡CH under the conditions specified (ozonolysis followed by reduction with Zn/H2O2) are acetic acid and formic acid.
Final Answer:
The product of the reaction is both acetic acid and formic acid.
- Acetic acid (CH3COOH)
- Formic acid (HCOOH)
The product of the reaction is both acetic acid and formic acid.
Topper's Solved these Questions
- HYDROCARBONBOOK - ERRORLESS CHAPTER - HYDROCARBONEXERCISE - ORDINARY THINKING (Objective question) (AROMATIC HYDROCARBON)
128 Videos
- HYDROCARBONBOOK - ERRORLESS CHAPTER - HYDROCARBONEXERCISE - CRITICAL THINKING (Objective questions)
24 Videos
- HYDROCARBONBOOK - ERRORLESS CHAPTER - HYDROCARBONEXERCISE - ORDINARY THINKING (Objective question) (ALKENE)
129 Videos
- HALOGEN CONTAINING COMPOUNDBOOK - ERRORLESS CHAPTER - HALOGEN CONTAINING COMPOUNDEXERCISE - Critical Thinking (Jee (Advanced) 2018)
1 Video
- IONIC EQUILIBRIUMBOOK - ERRORLESS CHAPTER - IONIC EQUILIBRIUMEXERCISE - JS Jee Section (JEE Advanced (Numeric answer type question))
1 Video
Similar Questions
Which among the following is very strong o-p-directing groups?
View SolutionIn the following reactions,
(a) H3C−CH3| CH−CH∣ OH−CH3H+/heat−−−−−→AMajorproduct+BMinorproduct
(b) AHBr,dark−−−−−−−−−−−−→in absence of peroxideCMajorproduct+DMinorproduct
The major products (A) and (C) are respectively:
View SolutionThe disappearance of the characteristic purple colour of KMnO4 in its reaction with an alkene is the test for unsaturation it is known as:
View SolutionList -I contains reaction and List-II contains major products. Match each reaction in List-I with one or more product in List-II
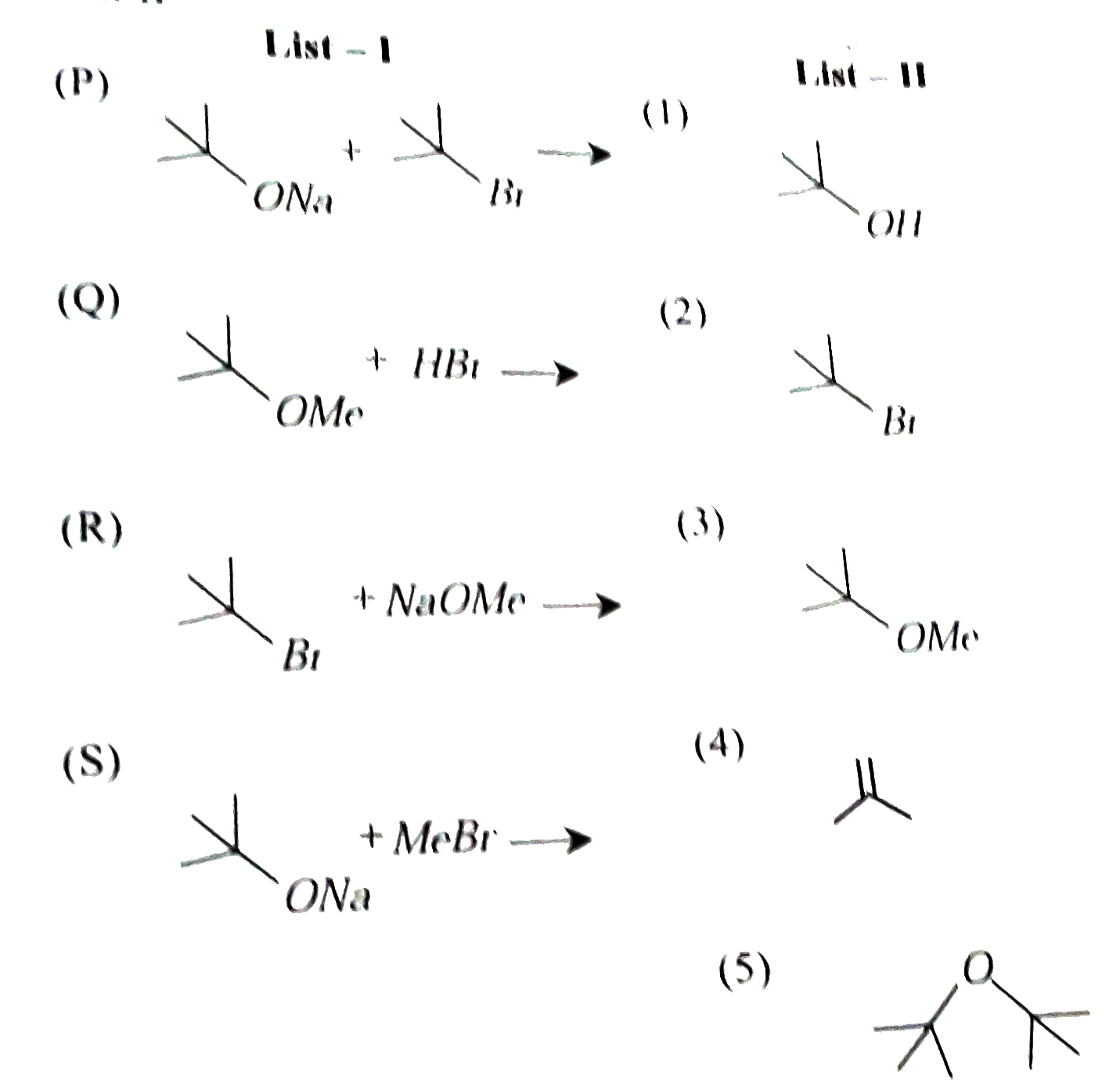
View SolutionThe solubility of a salt of weak acid (AB) at pH 3 is Y×10−3 mol L−1. The value of Y is ____. (Given that the value of solubility product of AB (Ksp)=2×10−10 and the value of ionization constant of HB (Ka)=1×10−8)
View Solutionनिम्नलिखित अभिक्रिया में उत्पाद है:
CH3−CH3∣∣C∣CH3−CH=CH2H2∅H+→
View SolutionWhat are the products formed in the reaction given below?
Ph−CH2−CH=CH−CH31)O3−−−−−−−→2)Zn+H2O?
View SolutionCH3−CH3∣C=CH−CH3∣C∣CH3−CH3NalO4/KMnO4−−−−−−−−−→
products. Products are:
View SolutionThe major products of the following reaction are :
CH3−CH3∣CH−CH−CH3∣OSO2CH3(i)KOtBu/Δ−−−−−−−−→(ii)O3/H2O2
View Solutionनिम्नलिखित अभिक्रिया का उत्पाद है
CH_3 C-= C CH_2 CH_3 underset("(2) जलअपघटन" ) overset((1) O_3)to
View SolutionCH3−C≡CHO3−−−−−→Zn/H2O2 उत्पाद, इस अभिक्रिया में बनने वाला उत्पाद है
View SolutionThe major products of the following reaction are :
CH3−CH3∣CH−CH−CH3∣OSO2CH3(i)KOtBu/Δ−−−−−−−−→(ii)O3/H2O2
View SolutionCH2=CH−CH=C∣CH3−CH3+HBr−−−→CH3−Br∣CH−CH=C∣CH3−CH3+CH3−CH(II)=CH−Br∣C∣CH3−CH3
Correct statement about the above reaction is
View SolutionH214C=CH−CH3low conc. ofBr2−−−−−−−−→or highi temp.(?)
Product of the above reaction is :
View SolutionCH3−CH2−CH2−C≡CHBH3−−→THFH2O2−−→OH−Identify the product
View Solution

