order of reactivity of C2H6,C2H4 and C2H2 is
C2H6>C2H4>C2H2
C2H2>C2H6>C2H4
C2H2>C2H4>C2H6
All are equally reactive
The correct Answer is:C
To determine the order of reactivity of the compounds C2H6, C2H4, and C2H2, we will analyze the structure and type of each compound:
1. Identify the Compounds:
- C2H6 is ethane, which is an alkane.
- C2H4 is ethylene, which is an alkene.
- C2H2 is acetylene, which is an alkyne.
2. Understand the Reactivity of Hydrocarbons:
- Alkanes (like ethane) are saturated hydrocarbons and generally have the lowest reactivity due to the presence of only single bonds.
- Alkenes (like ethylene) contain at least one double bond, which makes them more reactive than alkanes because the double bond can participate in addition reactions.
- Alkynes (like acetylene) contain at least one triple bond, which makes them even more reactive than alkenes due to the presence of two pi bonds that can participate in reactions.
3. Order the Reactivity:
- Since alkanes are the least reactive, we place C2H6 (ethane) at the lowest position in the reactivity order.
- Next, C2H4 (ethylene) is more reactive than C2H6 because of its double bond.
- Finally, C2H2 (acetylene) is the most reactive due to its triple bond.
4. Final Order of Reactivity:
- The order of reactivity from least to most reactive is:
C2H6<C2H4<C2H2
Final Answer: The order of reactivity is C2H6<C2H4<C2H2.
---
- C2H6 is ethane, which is an alkane.
- C2H4 is ethylene, which is an alkene.
- C2H2 is acetylene, which is an alkyne.
- Alkanes (like ethane) are saturated hydrocarbons and generally have the lowest reactivity due to the presence of only single bonds.
- Alkenes (like ethylene) contain at least one double bond, which makes them more reactive than alkanes because the double bond can participate in addition reactions.
- Alkynes (like acetylene) contain at least one triple bond, which makes them even more reactive than alkenes due to the presence of two pi bonds that can participate in reactions.
- Since alkanes are the least reactive, we place C2H6 (ethane) at the lowest position in the reactivity order.
- Next, C2H4 (ethylene) is more reactive than C2H6 because of its double bond.
- Finally, C2H2 (acetylene) is the most reactive due to its triple bond.
- The order of reactivity from least to most reactive is:
C2H6<C2H4<C2H2
Similar Questions
Knowledge Check
Order of reactivity of C2H6,C2H4andC2H2 is
AC2H6>C2H4>C2H2BC2H2>C2H6>C2H4CC2H2>C2H6DAll are equally reactiveThe descending order of reactivity of C2H6,C2H4,C2H2andC6H6 towards addition reaction is
AC2H4>C2H2>C6H6>C2H6BC2H2>C2H4>C6H6>C2H6CC6H6>C2H6>C2H4>C2H2DC2H56>C2H4>C2H2>C6H6The descending order of reactivity of C2H6,C2H4,C2H2andC6H6 towards addition reaction is
AC2H4>C2H2>C6H6>C2H6BC2H2>C2H4>C6H6>C2H6CC6H6>C2H6>C2H4>C2H2DC2H6>C2H4>C2H2>C6H6
Arrange the following alcohols in the increasing order of reactivity with HBr,
C6H5CH2OH,C6H5_(2)CHOH,(C6H6)3COH,p−ClC6H4CH2OH,p−NO2C6H4CH2OH
View SolutionDetermine ΔrH of the reaction
C2H6→C2H4+H2
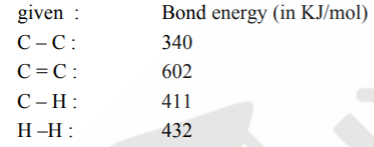
View SolutionIn SN2 reaction the order of reactivity of the halides. CH3X,C2H5X,n−C3H7X,n−C4H9X is
View SolutionUsing C2H5Cl/Na by Wurtz reaction in addition to C4H10,C2H4 and C2H6 are also formed. It is due to
View SolutionThe correct order of reactivity of the compounds
(I) C6H5H2(Br), (II) C6H5CH(C6H5)Br, (III) C6H5CH(CH3)Br, (IV) C6H5C(CH3)(C6H5)Br in the increasing order of reactivity in SN2 reactions is
View Solution

