Assertion :Activating groups are electron donors.
Reason: Nitroso group is activating group
If both assertion and reason are true and the reason is the correct explanation of the assertion
If both assertion and reason are true but reason is not the correct explanation of the assertion
if assertion is true but reason is false
If assertion and reason both are false
The correct Answer is:C
To solve the question, we need to analyze both the assertion and the reason provided.
Step 1: Analyze the Assertion
The assertion states that "Activating groups are electron donors."
- Activating groups are substituents on a benzene ring that increase the rate of electrophilic substitution reactions.
- Many activating groups, such as -NH2, -OH, and -OCH3, do indeed donate electron density to the benzene ring through resonance due to the presence of lone pairs of electrons.
- However, there are also groups like alkyl groups (e.g., -CH3) that do not have lone pairs but still activate the ring through hyperconjugation.
Conclusion for Assertion: While many activating groups are electron donors, it is not universally true for all activating groups. Therefore, the assertion is false.
Step 2: Analyze the Reason
The reason states that "Nitroso group is an activating group."
- The nitroso group (-NO) is known to be a deactivating group. It withdraws electron density from the benzene ring through resonance (due to its -M effect) and inductive effects.
- This means that the presence of a nitroso group will decrease the reactivity of the benzene ring towards electrophilic substitution, making it a deactivating group rather than an activating group.
Conclusion for Reason: The nitroso group is not an activating group; it is a deactivating group. Therefore, the reason is also false.
Final Conclusion
Both the assertion and the reason are false.
Answer
The correct answer is: Option 4: Both Assertion and Reason are false.
---
The assertion states that "Activating groups are electron donors."
- Many activating groups, such as -NH2, -OH, and -OCH3, do indeed donate electron density to the benzene ring through resonance due to the presence of lone pairs of electrons.
- However, there are also groups like alkyl groups (e.g., -CH3) that do not have lone pairs but still activate the ring through hyperconjugation.
The reason states that "Nitroso group is an activating group."
- This means that the presence of a nitroso group will decrease the reactivity of the benzene ring towards electrophilic substitution, making it a deactivating group rather than an activating group.
Both the assertion and the reason are false.
The correct answer is: Option 4: Both Assertion and Reason are false.
Topper's Solved these Questions
- HYDROCARBONBook:ERRORLESS Chapter:HYDROCARBONExercise:CRITICAL THINKING (Objective questions)
Explore 24 Videos
- HYDROCARBONBook:ERRORLESS Chapter:HYDROCARBONExercise:JEE SECTION (Only one choice correct answer)
Explore 93 Videos
- HYDROCARBONBook:ERRORLESS Chapter:HYDROCARBONExercise:ORDINARY THINKING (Objective question) (ALKYNE)
Explore 120 Videos
- HALOGEN CONTAINING COMPOUNDBook:ERRORLESS Chapter:HALOGEN CONTAINING COMPOUNDExercise:Critical Thinking (Jee (Advanced) 2018)
Explore 1 Video
- IONIC EQUILIBRIUMBook:ERRORLESS Chapter:IONIC EQUILIBRIUMExercise:JS Jee Section (JEE Advanced (Numeric answer type question))
Explore 1 Video
Similar Questions
In the following reactions,
(a) H3C−CH3| CH−CH∣ OH−CH3H+/heat−−−−−→AMajorproduct+BMinorproduct
(b) AHBr,dark−−−−−−−−−−−−→in absence of peroxideCMajorproduct+DMinorproduct
The major products (A) and (C) are respectively:
View SolutionMain constituent of marsh gas is
View SolutionCarbon-carbon bond length is minimum in
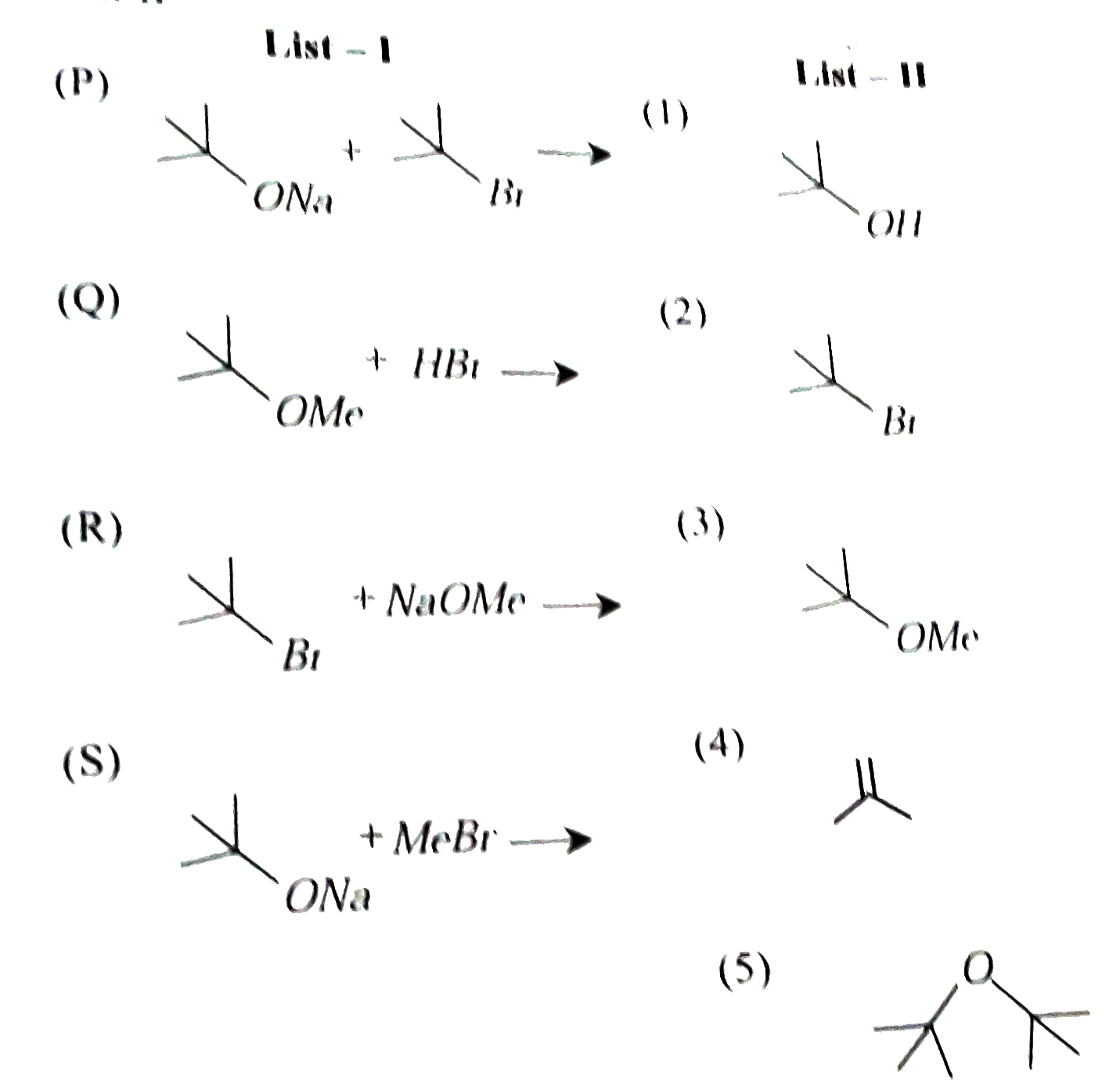
View SolutionList -I contains reaction and List-II contains major products. Match each reaction in List-I with one or more product in List-II
View SolutionThe solubility of a salt of weak acid (AB) at pH 3 is Y×10−3 mol L−1. The value of Y is ____. (Given that the value of solubility product of AB (Ksp)=2×10−10 and the value of ionization constant of HB (Ka)=1×10−8)
View SolutionIf aromatic ring is substituted by more than one group then electrophilic aromatic substitution reaction take place according to more activating group. The group which donates electrons to aromatic ring knwn as activating group and which withdraw electrons from the ring is called electron withdrawing group. generally all lectron releasing groups activates benzene ring towards electrophilic substitution and electron withdrawing groups deactivates ring towards electrophilic substitutions. Q. Find out major product of following reaction
View SolutionAssertion : Picric acid is a strong acid inspite of the absence of the carboxyl group.
Reason : The three −NO2 groups in picric acid activate the phenate ion.
View SolutionIn ether the active group is
View SolutionWhich of the following statement is /are correct ?
An activating group is an electron releasing group
2. An activating group activate all position of benzene ring
3. The effect of any group whether activating or decativating is the strongest at ortho and para position in the benzene ring
4. An activating group activate only the ortho and para position in benzing ring
View SolutionWhat are activating and deactivating groups ?
View SolutionFor the following Assertion and Reason the correct option is: Assertion: For hydrogenation reactions, the catalytic activity increases from Group 5 to Group 11 metals with maximum activity shown by Group 7-9 elements. Reason: The reactants are most strongly adsorbed on group 7-9 element
View SolutionThe group which is most activating is
View SolutionWhat are activating and deactivatikn groups
View Solutionप्रक्कथन : सक्रिय समूह इलेक्ट्रॉन दाता होते हैं।
कारण :नाइट्रोसो समूह सक्रिय समूह है।
View SolutionAssertion : Picric acid is a strong acid inspite of the absence of the carboxyl group.
Reason : The three −NO2 groups in picric acid activate the phenate ion.
View Solution
