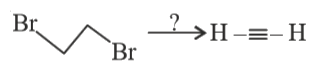In which one of the following conversions phosphorus pentachloride is used as a reagent?
A
H2C=CH2→CH3CH2Cl
B
H3C−O−CH3→CH3Cl
C
CH3CH2OH→CH3CH2Cl
D
HC≡CH→CH2=CHCl
Video Solution
Text Solution
The correct Answer is:B, C
CH3−O−CH3+PCl5→2CH3Cl+POCl3
CH3CH2OH+PCl5→CH3CH2Cl+HCl+POCl3
So, both reaction carried out by PCl5.
|
Similar Questions
Knowledge Check
Phosphorus pentachloride is ionic in
Avapour stateBliquid stateCsolid stateDall the three statesThe reagents for the following conversion
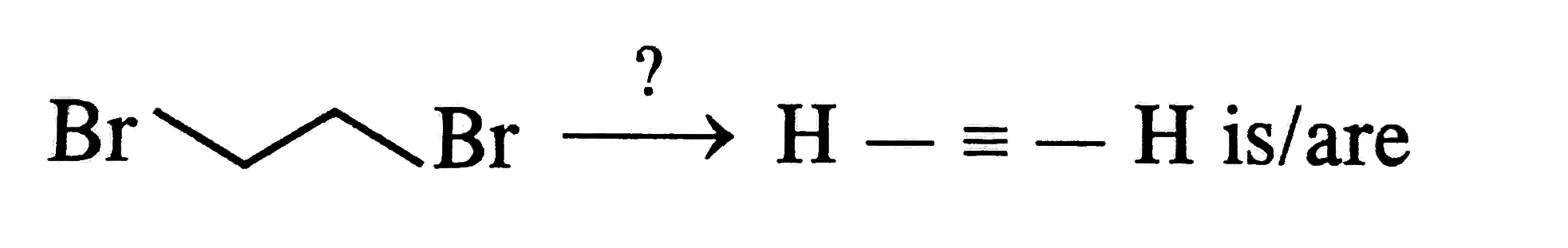 Aalcoholic KOHBalcholic KOH followed by NaNH2Caquesous KOH followed by NaNH2DZn/CH3OH
Aalcoholic KOHBalcholic KOH followed by NaNH2Caquesous KOH followed by NaNH2DZn/CH3OHPhosphorus pentachloride in the solid exists as :
APCl5BPCl+4Cl−CPCl+4PCl−6DPCl5.Cl2
Dissociation of phosphorus pentachloride is favoured by -
View SolutionThe reagents for the following conversion is/are

View SolutionThe reagent(s) for the following conversion is/are
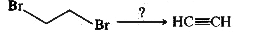
View SolutionThe reagent(s) for the following conversion is/are
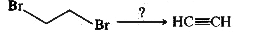
View SolutionThe reagents (s) for the following conversion
 CH≡CH .
CH≡CH .
View Solution