A substance C4H10O yields on oxidation a compound C4H8O which gives an oxime and a positive iodoform test. The original substance on treatment with conc. H2SO4 gives C4H8 . The structure of the compound is
CH3CH2CH2CH2OH
CH3Ch(OH)CH2CH3
(CH3)3COH
CH3CH2−O−CH2CH3
The correct Answer is:A
To solve the problem step by step, we need to analyze the given information about the substance with the molecular formula C4H10O and its reactions.
Step 1: Identify the Original Compound
The original compound has the formula C4H10O. This suggests it could be an alcohol or an ether. Since it yields a compound upon oxidation, it is likely an alcohol.
Step 2: Determine the Oxidation Product
Upon oxidation, the compound yields C4H8O. This indicates that the original compound is likely a secondary alcohol, which can be oxidized to a ketone.
Step 3: Analyze the Oxime Formation
The product C4H8O gives an oxime when treated with hydroxylamine. This suggests that the oxidation product is a ketone, as only ketones and aldehydes can form oximes.
Step 4: Positive Iodoform Test
The fact that the oxidation product gives a positive iodoform test indicates that it contains a methyl ketone group (i.e., a structure like RCOCH3).
Step 5: Original Compound's Reaction with Concentrated H2SO4
The original substance, when treated with concentrated sulfuric acid, yields C4H8. This suggests a dehydration reaction, which is typical for alcohols, leading to the formation of an alkene.
Step 6: Identify the Structure of the Original Compound
Given that the original compound is likely a secondary alcohol that can yield a methyl ketone upon oxidation, we can deduce that the original compound is 2-butanol. The oxidation of 2-butanol leads to butan-2-one (a methyl ketone), which gives a positive iodoform test and can form an oxime.
Conclusion
The structure of the original compound C4H10O is 2-butanol.
Final Answer
The structure of the compound is:
2-butanol
---
The original compound has the formula C4H10O. This suggests it could be an alcohol or an ether. Since it yields a compound upon oxidation, it is likely an alcohol.
Upon oxidation, the compound yields C4H8O. This indicates that the original compound is likely a secondary alcohol, which can be oxidized to a ketone.
The product C4H8O gives an oxime when treated with hydroxylamine. This suggests that the oxidation product is a ketone, as only ketones and aldehydes can form oximes.
The fact that the oxidation product gives a positive iodoform test indicates that it contains a methyl ketone group (i.e., a structure like RCOCH3).
The original substance, when treated with concentrated sulfuric acid, yields C4H8. This suggests a dehydration reaction, which is typical for alcohols, leading to the formation of an alkene.
Given that the original compound is likely a secondary alcohol that can yield a methyl ketone upon oxidation, we can deduce that the original compound is 2-butanol. The oxidation of 2-butanol leads to butan-2-one (a methyl ketone), which gives a positive iodoform test and can form an oxime.
The structure of the original compound C4H10O is 2-butanol.
The structure of the compound is:
2-butanol
Similar Questions
Knowledge Check
A substance C44H16O yields on oxidation a compound C4H8O which gives an oxime and a positive iodoform test. The original substance on treatment with conc. H2SO4 gives C4H8 . The structure of the compound is
ACH3CH2CH2CH2OHBCH3CH(OH)CH2CH3C(CH3)3COHDCH3CH2−O−CH2CH3A substance C4H10O yields on oxidation a compound C4H8O which gives an oxime and a positive iodoform test. The original substance on treatment with conc. H2SO4 gives C4H8, The structure of the compound is
ACH3CH2CH2CH2OHBCH3CHOHCH2CH3C(CH3)3COHDCH3CH2−O−CH2CH3A substance C4H10O yield on oxidation C4H5O which gives oxime and positive iodoform test.
The original substance on treatment with conc. H2SO4 gives C4H8. The structure of compound isA B
B C
C D
D
A compound (X) C4H8O Which gives a 2,4-DNP derivative and a positive iodoform test is:
View SolutionCompound 'X' C4H8O which gives 2,4-DNP derivative and negative iodoform test is :
View SolutionGiven are the isomers of C8H8O2.
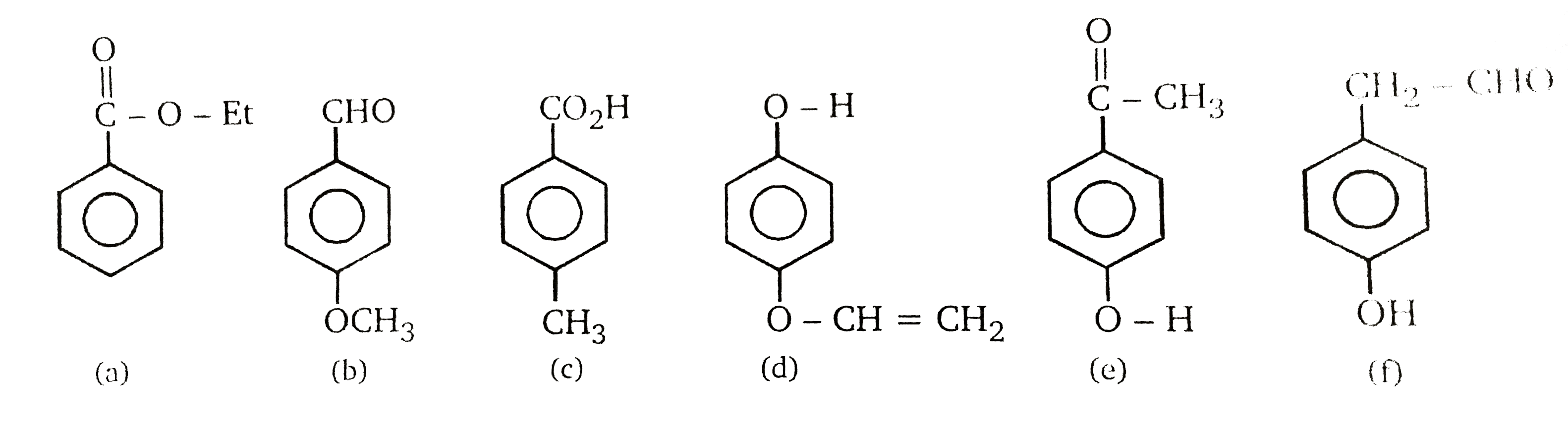
Which isomer gives positive iodoform test?
View SolutionC7H10O reacts with CH3MgBr r to give a compound C8H10O which gives the test with iodoform, than fine out structure of A
View SolutionAn compound (A ) C4H8Cl2 on alkaline hydrolysis to give compound (B ) C4H8O which gives an oxime and positive Tollen's reagent test . What is the structure of (A) ?
View Solution
