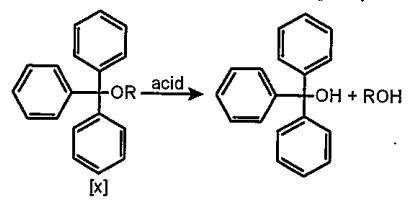
The acidic hydrolysis of ether (X) shown below is fastest when.
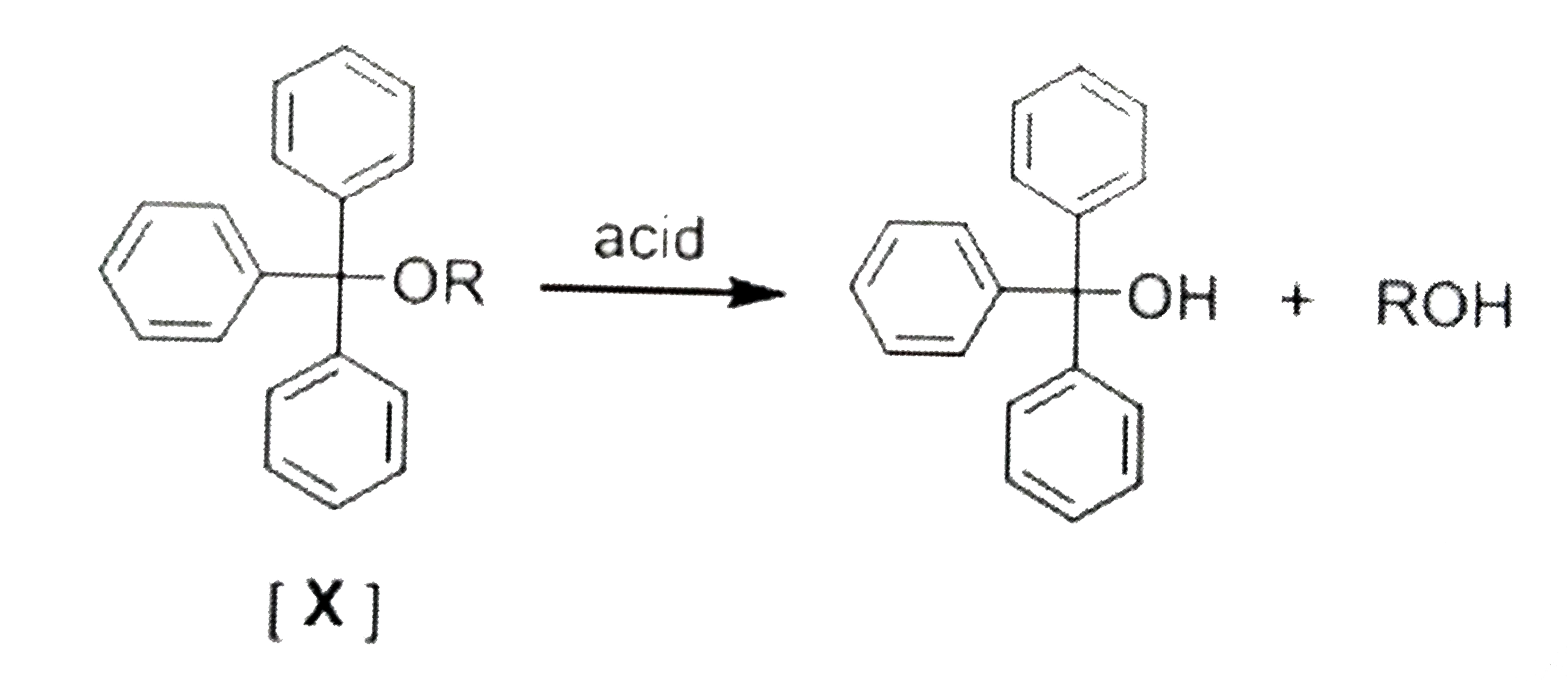
A
One phenyl group is replaced by a methyl group
B
One phenyl group is replaced by a para-methoxyphenyl group
C
Two phenyl groups a replaced by two paramethoxyphenyl groups
D
No structure change is made to X
Video Solution
Text Solution
The correct Answer is:C
Ph3C−ORH⊕−−→H2OPh3C−OH+ROH
The reaction proceeds by SN1 Mechanism
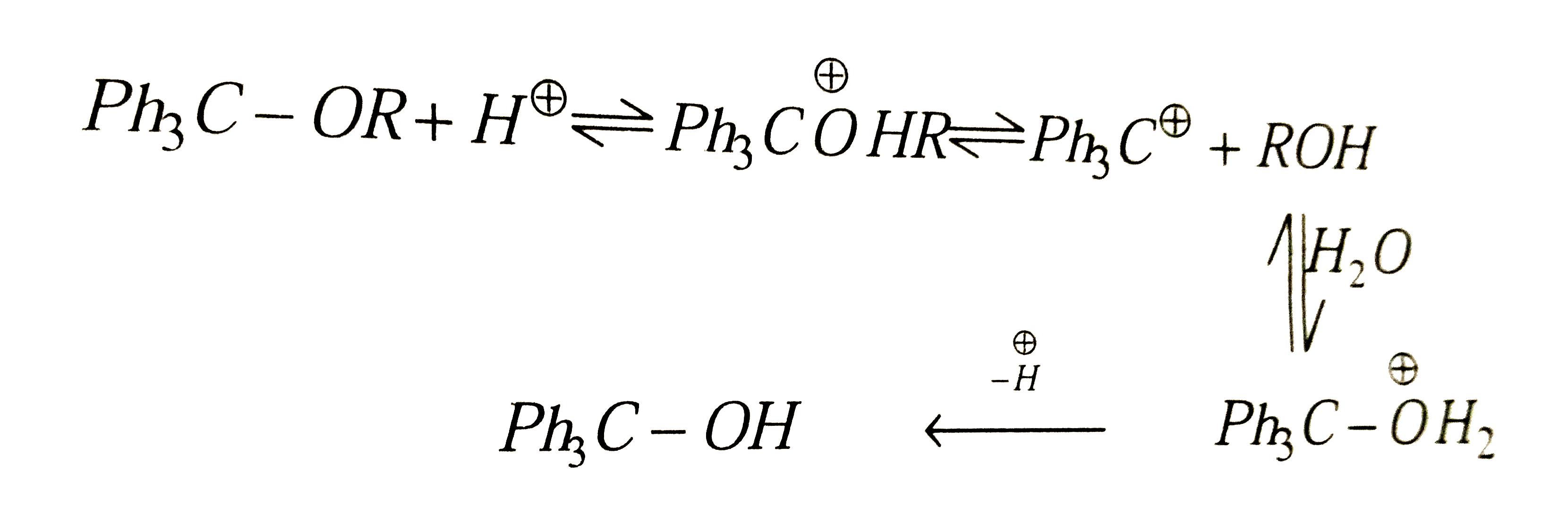
Greater the electron releasing effect of the attached groups greater is the stability of intermediate carbocation , and faster is the rate of reaction.
If two pj- groups are replaced by MeO 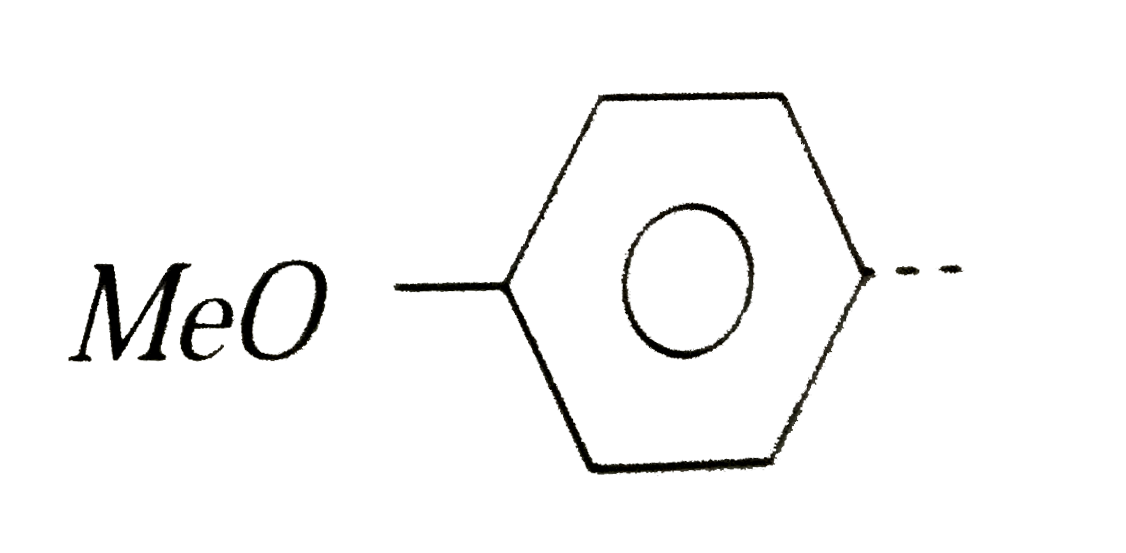
groups , strong +M effect of MeO-groups stabilize, the carbocation better there by making the reaction faster.
|
Similar Questions
The acidic hydrolysis of ether (X) shown below is fastest when.

View SolutionAcidic hydrolysis of ether gives
View SolutionPHYSICAL PROPERTIES OF ETHER|SOLUBILITY| BOILING POINT| REACTIONS OF ETHER WITH HALIDES| ACIDS| HYDROLYSIS OF ETHERS
View Solutionनीचे दिए ईथर (X) का अम्लीय जल - अपघटन तीव्रतम वेग से होता है जब
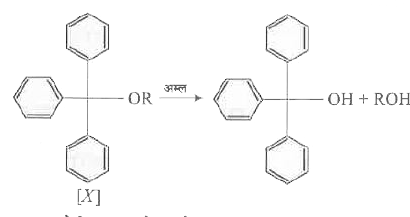
View SolutionThe reaction is fastest when X is :
View Solutionनीचे दिए ईथर (X) का अम्लीय जल - अपघटन तीव्रतम वेग से होता है जब

View SolutionThe acidic hydrolysis of ether (X)shown below is fastest when
View SolutionThe acidic hydrolysis of ether (X)shown below is fastest when
View Solution
