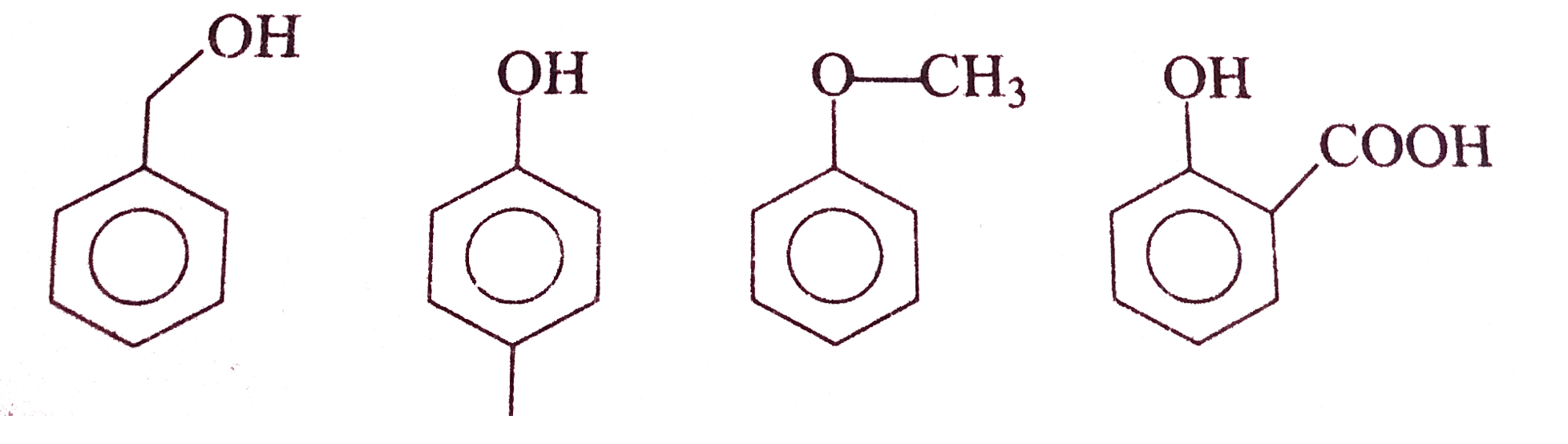
Which of the following will give characteristic colour with FeCl3
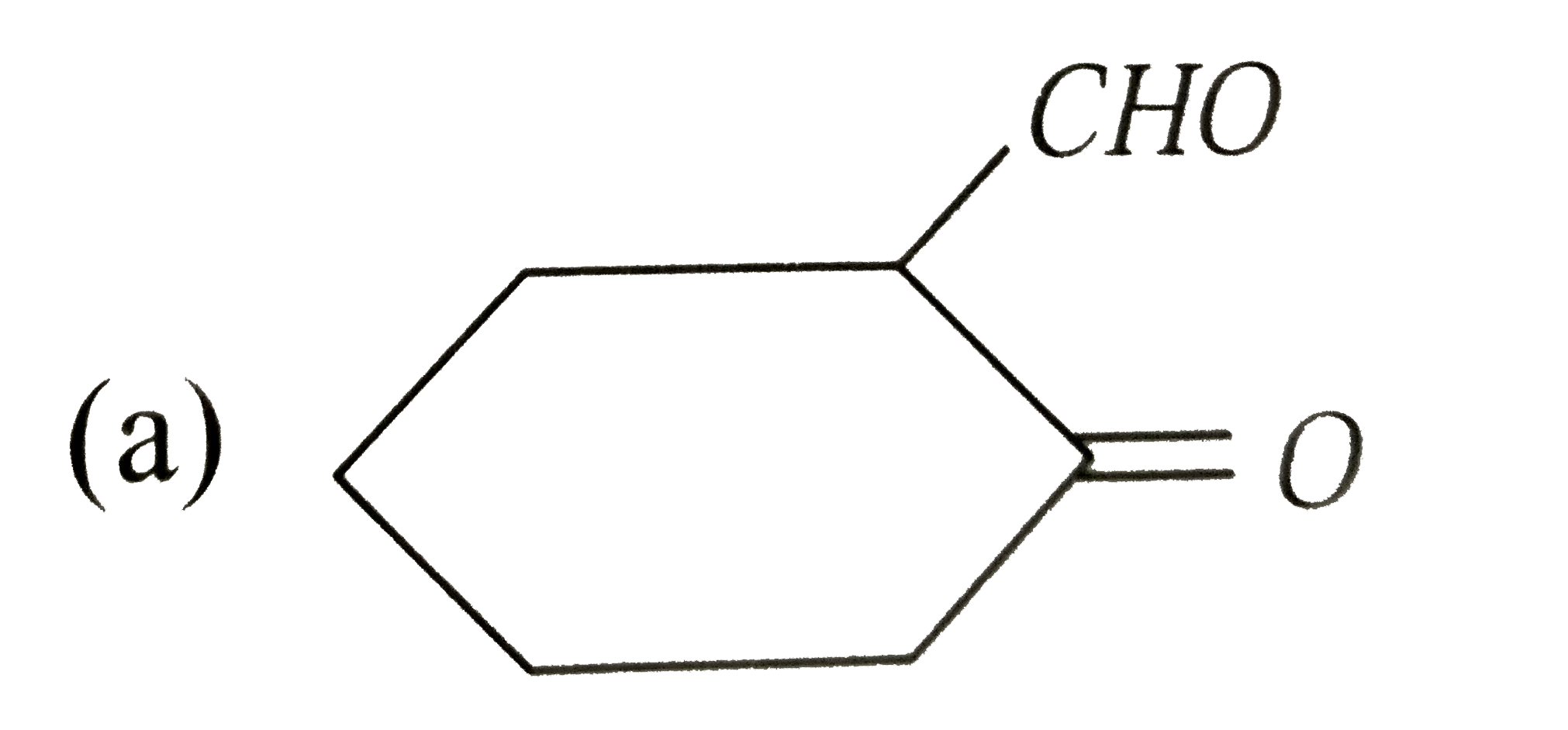
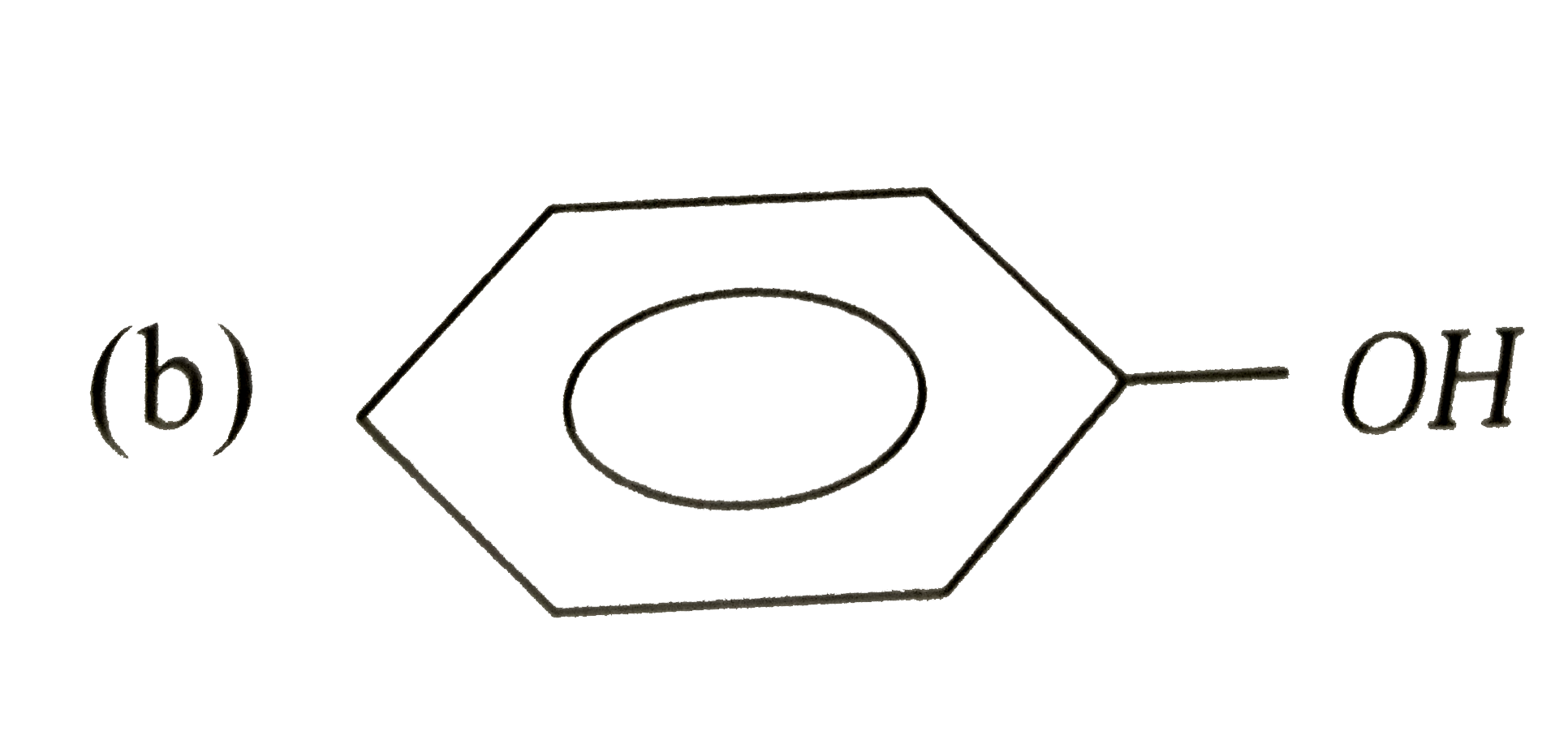
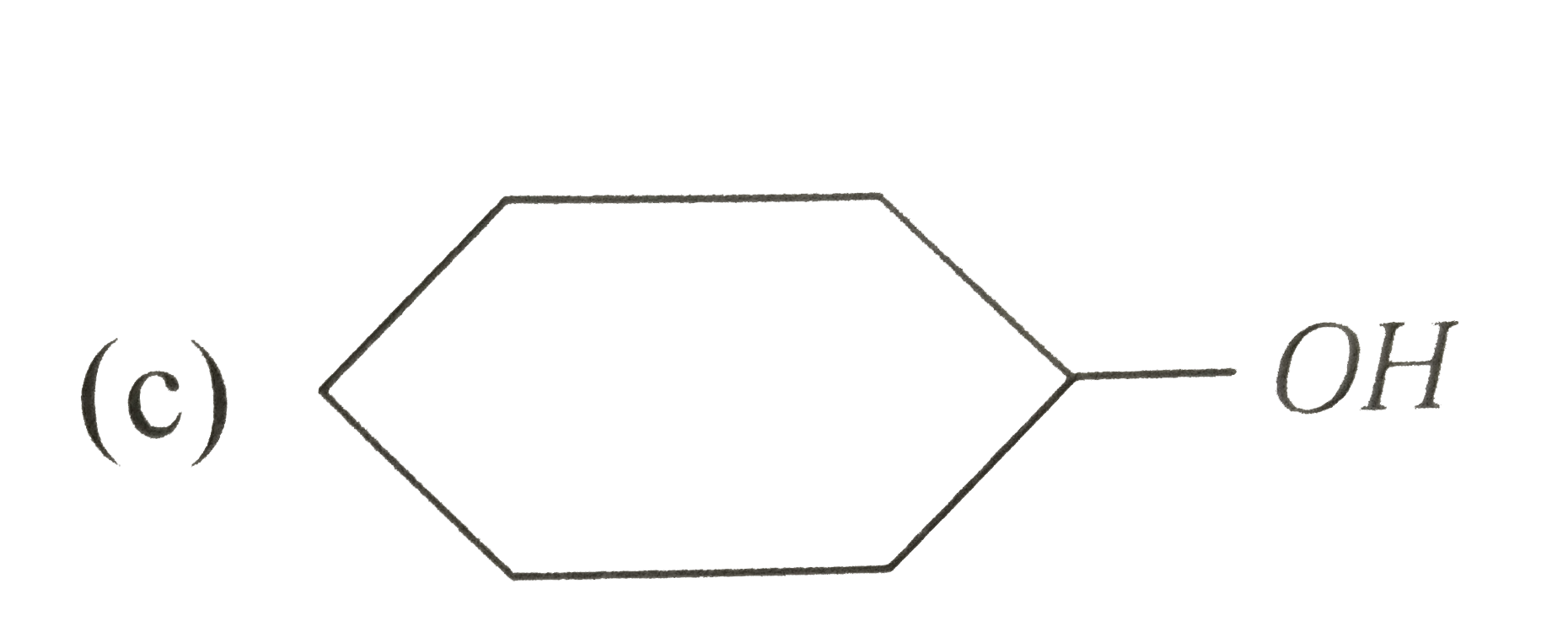
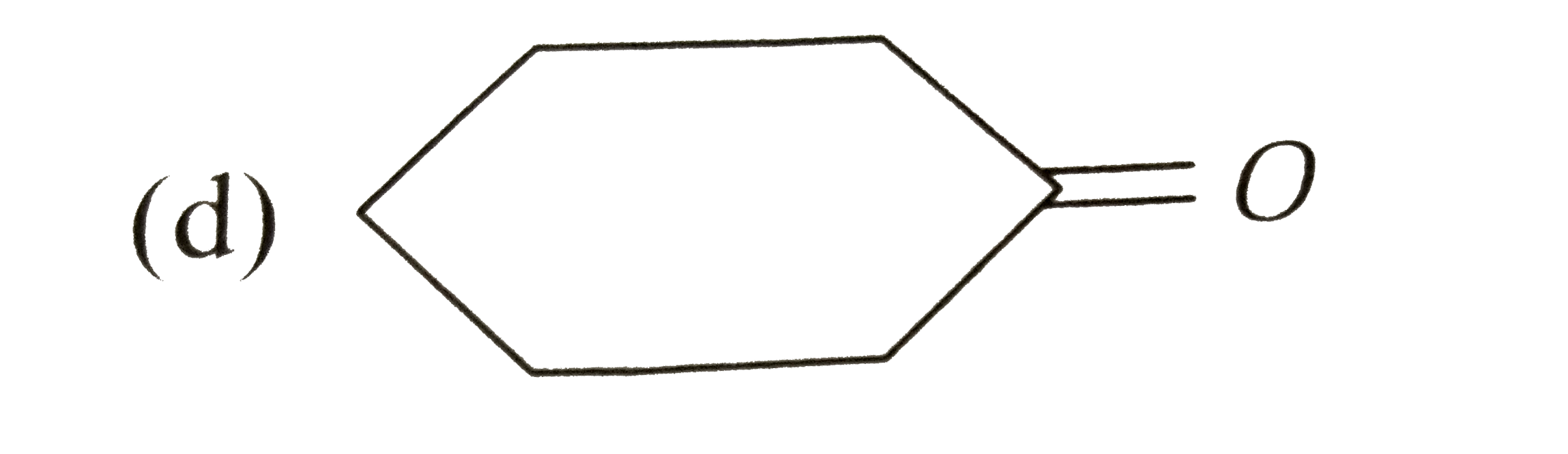
The correct Answer is:A, B
To determine which of the given compounds will give a characteristic color with FeCl₃, we need to analyze the options provided and their chemical nature.
1. Understanding the Reaction with FeCl₃:
- Ferric chloride (FeCl₃) is known to react with phenolic compounds to form colored complexes. The most common color observed is violet.
2. Identifying the Compounds:
- We need to identify which of the given options are phenols or enols, as these are the compounds that will react with FeCl₃ to produce a color.
3. Analyzing Each Option:
- Option 1: Check if it is a phenol or enol. If it is not, it will not give a characteristic color with FeCl₃.
- Option 2: This is identified as a phenol. Since phenols react with FeCl₃ to give a violet color, this option is likely to be correct.
- Option 3: Similar to option 1, check if it is a phenol or enol. If it is not, it will not react with FeCl₃.
- Option 4: Again, check if it is a phenol or enol. If it is not, it will not give a characteristic color.
4. Conclusion:
- After analyzing the options, we find that Option 2, which is a phenol, will give a characteristic violet color with FeCl₃. The other options do not have the required functional groups to react with FeCl₃.
Final Answer:
- The compound that will give a characteristic color with FeCl₃ is Option 2 (the phenol).
---
- Ferric chloride (FeCl₃) is known to react with phenolic compounds to form colored complexes. The most common color observed is violet.
- We need to identify which of the given options are phenols or enols, as these are the compounds that will react with FeCl₃ to produce a color.
- Option 1: Check if it is a phenol or enol. If it is not, it will not give a characteristic color with FeCl₃.
- Option 2: This is identified as a phenol. Since phenols react with FeCl₃ to give a violet color, this option is likely to be correct.
- Option 3: Similar to option 1, check if it is a phenol or enol. If it is not, it will not react with FeCl₃.
- Option 4: Again, check if it is a phenol or enol. If it is not, it will not give a characteristic color.
- After analyzing the options, we find that Option 2, which is a phenol, will give a characteristic violet color with FeCl₃. The other options do not have the required functional groups to react with FeCl₃.
- The compound that will give a characteristic color with FeCl₃ is Option 2 (the phenol).
Similar Questions
Knowledge Check
Which of the following gives characteristic deep colour with FeCl3 ?
AEthanolBResorcinolCPhenolDBoth "b" and "c"Phenol gives characteristic colouration with
Aisodine solutionBbromine waterCammonium hydroxideDaqueous ferric chloride solutionWhich of the following will give blood red colour with FeCl5 in sodium extract?
ANH2CONH2BNH2CSNH2CCH5NHNH2DCH3C≡N
Which of the following will give blood red colour with FeCl5 in sodium extract?
View SolutionPhenol gives characteristic colouration with
View SolutionWhich of the following compounds will give a characteristic deep colour with ferric chloride solution ?
View SolutionWhich of the following can give purple colour with neutral FeCl3
View SolutionWhich of the following can give purple colour with neutral FeCl3

View Solution

