The mole fraction of a solute in a solutions is 0.1. At 298K molarity of this solution is the same as its molality. Density of this solution at 298 K is 2.0gcm−3. The ratio of the molecular weights of the solute and solvent, MWsoluteMWsolvent is
The correct Answer is:9
Mole fraction of solute 0.1 means that 1 mol of solution has 0.1 mol of solute and 0.9 mol of solvent .
If M1 is molar mass of solute and M2 is molar mass of solvent , then
Mass of solvent =0.9×M2
Molality =0.10.9M2×100
Volume of solution =Mass of solute + Mass of solvent Density
=0.1M1+0.9M22
Molarity =0.1(0.1M1+0.9M2)×2×1000
Since molality = molarity .
0.10.9M2×1000=0.1×2×10000.1M1+0.9M2
1000.9M2=2000.1M1+0.9M2
10M1+90M2=180M2
10M1=90M2 ∴ M1M2=9
Topper's Solved these Questions
- SOLUTIONSBook:MODERN PUBLICATIONChapter:SOLUTIONSExercise:UNIT PRACTICE TEST
Explore 14 Videos
- SOLUTIONSBook:MODERN PUBLICATIONChapter:SOLUTIONSExercise:COMPETITION FILE (MARTIX MATCH TYPE QUESTIONS)
Explore 2 Videos
- SOLID STATEBook:MODERN PUBLICATIONChapter:SOLID STATEExercise:UNIT PRACTICE TEST
Explore 13 Videos
- SURFACE CHEMISTRYBook:MODERN PUBLICATIONChapter:SURFACE CHEMISTRYExercise:COMPETITION FILE (OBJECTIVE TYPE QUESTIONS) (MATCHING LIST TYPE QUESTIONS)
Explore 2 Videos
Similar Questions
The vapor pressure of acetone at 20∘C is 185 torr. When 1.2g of a non-volatile solute was dissolved in 100g of acetone at 20∘C, it vapour pressure was 183 torr. The molar mass (gmol−1) of solute is:
View SolutionMatch the behaviour of solution in Column I with the example listed in Column II.

View SolutionThe ratio of closed packed atoms to tetrahedral holes in cubic close packing is :
View SolutionMatch list I with list II and select the correct answer using the code :
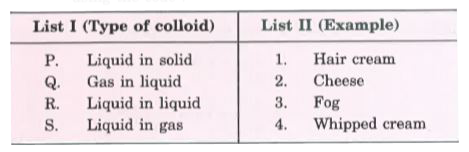
View SolutionThe expression relating mole fraction of solute (χ2) and molarity (M) of the solution is: (where d is the density of the solution in gL−1 and Mw1 and Mw2 are the molar masses of solvent and solute, respectively
View SolutionThe molality of a solute in a solvent (molar mass, 20g mol^(-1)) is 0.1 mol kg^(-1) . What is the mole fraction of the solute?
View SolutionIf
m= molality of solution (molKg−1)
χsolute= mole fraction of solute
Msolvent= molar mass of solvent (gmol−1)
View SolutionThe mole fraction of a solute in a solutions is 0.1. At 298K molarity of this solution is the same as its molality. Density of this solution at 298 K is 2.0gcm−3. The ratio of the molecular weights of the solute and solvent, MWsoluteMWsolvent is
View SolutionA solution is made by dissolving CaBr2 in water (solvent) such that mass fraction of solute and solvent is same in the solution. The molality of solution is :
View SolutionThe mole fraction of a solute in a solutions is 0.1. At 298K molarity of this solution is the same as its molality. Density of this solution at 298 K is 2.0gcm−3. The ratio of the molecular weights of the solute and solvent, MWsoluteMWsolvent is
View SolutionIf the density of the solvent is 2.5 kg dm^(-3) . The 2 molal solution of a solute in this solvent will be molal solution of a solution of a solute in this solvent will be
View SolutionThe mole fraction of a solute in a solution is 0.1. At 298 K, molarity of this solution is same as its molality Density of the solution at 298 K is "2.0 g cm"^(-3) . The ratio of the molecular weights of the solute and solvent ((MW_("solute"))/(MW_("solvent"))), is
View SolutionThe mole fraction of a solute in a solutions is 0.1. At 298K molarity of this solution is the same as its molality. Density of this solution at 298 K is 2.0gcm−3. The ratio of the molecular weights of the solute and solvent, MWsoluteMWsolvent is
View Solutionएक विलेय का एक विलयन में मोल भिन्न 0.1 है। 298 K पर इस विलयन की मोलरता इसकी मोललता के समान है। इस विलयन का घनत्व 298 K पर 2.0 ग्राम "सेमी"^(-3) है। विलेय तथा विलायक के अणुभारों का अनुपात ("अणुभार"_("विलेय")/("अणुभार"_("विलयाक") है।
View SolutionThe mole fraction of solvent in 0.1 molal aqueous solution is
View Solutionएक विलेय का एक विलयन में मोल भिन्न 0.1 है। 298 K पर इस विलयन की मोलरता इसकी मोललता के समान है। इस विलयन का घनत्व 298 K पर 2.0 ग्राम "सेमी"^(-3) है। विलेय तथा विलायक के अणुभारों का अनुपात ("अणुभार"_("विलेय")/("अणुभार"_("विलयाक") है।
View Solution
