For the dissociation equilibrium,
N2O4(g)⇔2NO2(g), the variation of free energy with the fraction of N2O4 dissociated under standard conditions is shown in the figure :
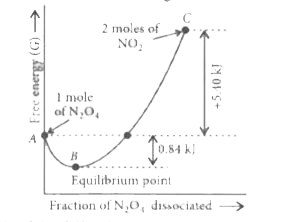
Which of the following statements is/are correct?
The free energy change for the forward reaction is negative
The free energy change for the backward reaction is negative
The net free energy change for the complete reaction is positive
Forward reaction is more spontaneous than backward reaction
The correct Answer is:A, B, C
ΔG∘ for conversion of 1 mole of N2O4 into equilibrium mixture (forward reaction)
=−0.84kJ,i.e.,−ve
ΔG∘ for conversion of 1 mole of N2O4 completely into 2 moles of NO2=+5.40kJ.
ΔG∘ for complete conversion is positive therefore, complete conversion is not possible.
As ΔG∘ for backward reaction is more negative than for forward reaction, i.e., formation of N2O4 is more spontaneous.
Topper's Solved these Questions
- CHEMICAL EQUILIBRIA BOOK - MTG-WBJEECHAPTER - CHEMICAL EQUILIBRIA EXERCISE - WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 1 : Single Option Correct Type )
4 Videos
- CHEMICAL EQUILIBRIA BOOK - MTG-WBJEECHAPTER - CHEMICAL EQUILIBRIA EXERCISE - WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 2 : Single Option Correct Type )
1 Video
- CHEMICAL EQUILIBRIA BOOK - MTG-WBJEECHAPTER - CHEMICAL EQUILIBRIA EXERCISE - WB JEE WORKOUT (CATEGORY 2 : Single Option Correct Type )
15 Videos
- CHEMICAL ENERGETICSBOOK - MTG-WBJEECHAPTER - CHEMICAL ENERGETICSEXERCISE - WB JEE PREVIOUS YEARS QUESTIONS (ONE OR MORE THAN ONE OPTION CORRECT TYPE )
2 Videos
- CHEMISTRY IN INDUSTRYBOOK - MTG-WBJEECHAPTER - CHEMISTRY IN INDUSTRYEXERCISE - WB JEE PREVIOUS YEARS QUESTIONS (SINGLE OPTION CORRECT TYPE)
1 Video
Similar Questions
For the reaction 2SO2(g)+O2(g)⇔2SO3(g) at 300 K, the value of ΔG∘ is −690.9R. The equilibrium constant value for the reaction at that temperature is (R is gas constant)
View SolutionThe standard Gibbs free energy change (ΔG∘) at 25∘C for the dissociation of N2O4(g) to NO2(g) is
(given, equilibrium cont. =0.15,R=8.314JK−1mol−1)
View SolutionThe decomposition of N2O4 to NO2 is carried out at 280∘C in chloroform. When equilibrium is reached, 0.2 mol of N2O4 and 2×10−3 mol of NO2 are present in 2 litre solution. The equilibrium constant for the reaction, N2O4⇔2NO2 is
View SolutionFor a spontaneous process, the correct statement(s) is
View SolutionNitric acid can be obtained from ammonia via the formation of the intermediate compounds
View SolutionN2O4 dissociates as
N2O4(g)⇔2NO2(g)
At 40∘C and one atmosphere % decomposition of N2O4 is 50.3%. At what pressure and same temperature, the equilibrium mixture has the ratio of N2O4:NO2 as 1:8?
View SolutionThe degree of dissociation of N2O4(1) obeying the equilibrium,
N2O4(g)⇔2NO2(g), is approximately related to the pressure at equilibrium by :
View SolutionConsider the following equilibrium
N2O4(g)⇔2NO2(g)
Then the select the correct graph , which shows the variation in concentratins of N2O4 Against concentrations of N2O_(4):
View SolutionVapour density of the equilibrium mixture of NO_(2) "and" N_(2)O_(4) is found to be 38.33 . For the equilibrium N_(2)O_(4)(g)hArr2NO_(2)(g) . Calculatedegree of dissociation.
View SolutionAt the equilibrium of the reaction , N_(2)O_(4)(g)rArr2NO_(2)(g) , the observed molar mass of N_(2)O_(4) is 77.70 g . The percentage dissociation of N_(2)O_(4) is :-
View Solution18.4 g of N_(2)O_(4) is taken in a 1 L closed vessel and heated till the equilibrium is reached. N_(2)O_(4(g))rArr2NO_(2(g)) At equilibrium it is found that 50% of N_(2)O_(4) is dissociated . What will be the value of equilibrium constant?
View SolutionIf in the reaction, N_(2)O_(4)(g)hArr2NO_(2)(g), alpha is the degree of dissociation of N_(2)O_(4) , then the number of moles at equilibrium will be
View SolutionDuring thermal dissociation, the observed vapour density of N_(2)O_(4)(g) is 26.0. The extent of dissociation of N_(2)O_(4) is:
View SolutionAt the equilibrium of the reaction N_(2)O_(4) (g) rarr 2NO_(2)(g) the observed molar mass of N_(2) O_(4) is 77.7 gm . The percentage dissociation of N_(2)O_(4) is
View SolutionThe value of Kp for the equilibrium reaction
N2O4(g)<⇒2NO2(g) is 2, Calculate the percentage dissociation of N2O4 (g) at a pressure of 0.5 atm.
View Solution

