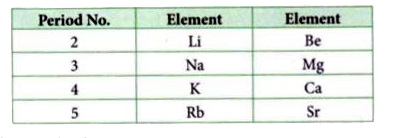
The position of eight elements in the Modern Periodic Table is given below where atomic numbers of elements are given in the parenthesis.

(i) Write the electronic configuration of Ca. (ii) Predict the number of valence electrons in Rb. i (iii) What is the number of shells in Sr? (iv) Predict whether K is a metal or a non-metal. (v) Which one of these elements has the largest atom in size? (vi) Arrange Be, Ca, Mg and Rb in the increasing order of the size of their respective atoms.
(i) Electronic configuration of Ca - 2, 8, 8, 2
(ii) Valence electrons in Rb is 1.
(iii) Five
(iv) Metal
(v) Rb is biggest in size.
(vi) Be < Mg < Ca < Rb
Similar Questions
Predict the number of valence electrons in Rb.
View SolutionThe atomic number of an element is 16. Predict whether it is a metal or a non-metal
View SolutionWrite the electronic configuration of the element with atomic number 102.
View SolutionThe atomic number of an element is 16. Predict the number of valence electrons in its atom
View SolutionWrite the electronic configurations and predict the number of unpaired electrons in the following :
(i) Cr(Z=24)
(ii) Fe(Z=26)
View SolutionThe atomic number of an element is 16. Predict its valency
View SolutionAtomic number of a few elements are given below 10, 20, 7, 14
What would be the electronic configuration for each of these elements?
View SolutionAtomic number of an element is 16. Predict.
(i) the number of valence electrons in its atom (ii) its valency
(iii) its group number (iv) whether it is a metal or a non-metal
(v) the nature of oxide formed by it. (vi) the formula of the chloride.
View SolutionWrite the electronic configuration of the element with atomic number 17. Indicate the valency of the element.
View SolutionWhat is the number of valence electrons in the atom of an element A having atomic number 20? Name the valence shell of this atom.
View Solution