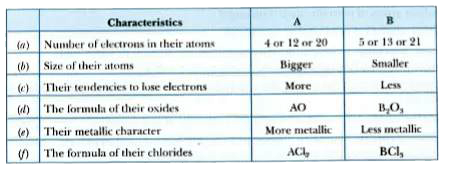
Two elements ‘A’ and 'B' belong to the 3rd period of Modern periodic table and are in group 2 and 13 respectively. Compare their following characteristics in tabular form:
(a) Number of electrons in their atoms (b) Size of their atoms (c) Their tendencies to lose electrons (d) The formula of their oxides (e) Their metallic character The formula of their chlorides
Similar Questions
Two elements 'P' and 'Q' belong to the same period of the modern periodic table and
are in Group-1 and Group-2 respectively. Compare their following characteristics in
tabular from:
(a) The number of electrons in their atoms
(b) The sizes of their atoms
(c) Their metallic characters
(d) Their tendencies to lose electrons
(e)The formula of their oxides
(f) The formula of their chlorides
View SolutionTwo elements 'P' and 'Q' belong to the same period of the modern periodic table and
are in Group-1 and Group-2 respectively. Compare their following characteristics in
tabular from:
(a) The number of electrons in their atoms
(b) The sizes of their atoms
(c) Their metallic characters
(d) Their tendencies to lose electrons
(e)The formula of their oxides
(f) The formula of their chlorides
View Solution
Knowledge Check
An element X belongs to group 2 and period 3 of the periodic table. The chemical formulae of its nitrate, sulphate and phosphate respectively will be
AX(NO3)2,XSO4,X3(PO4)2BXNO3,XSO4,XPO4CX3(NO3)2,X2(SO4)2,X2(PO4)3DX(NO3)3,X2(SO4)3,X2(PO4)3
Two elements X and Y belong to group 1 and 2 respectively in the same period. Compare them with respect to:
(a) the number of valence electrons (b) valency (c) metallic character (d) size of the atoms (e) formulae of their oxides and chlorides.
View SolutionTwo elements A and B belong to group 1 and 2 respectively in the same period. Compare them with respect to
the number of valence electrons
View SolutionNa, Mg and Al are the elements of the 3rd period of the Modern Periodic Table having group number 1, 2 and 13 respectively. Which one of these elements has the
maximum chemical reactivity ?
View SolutionTwo elements X and Y belong to groups 1 and 2 respectively in the same period. Compare them with respect to (a) the number of electrons in the outermost shell, (b) the number of valence electrons , (c) metallic character, (d) size of the atoms and ( e) formulae of the oxdies , hydrides and chlorides.
View SolutionTwo elements X and Y belong to group 1 and 2 respectively in the same period of periodic table. Compare these elements with respect to
(i) number of electrons in their outermost orbits (ii) their valencies
(iii) metallic character (iv) their atomic size
(v) formula of their chlorides (vi) formula of their sulphates
View SolutionTwo elements M and N belong to group I and II respectively and are in the same period of the periodic table. How do following properties of M and N vary ?
(a) Size of their atoms (b) Their metallic character
(c) Their valencies in forming oxides (d) Formulae of their chlorides
View SolutionAn element B belongs to the second period and Group 13. Give the formula of the oxide.
View Solution