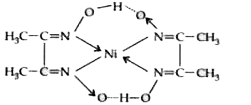
In basic medium the amount of Ni2+ in a solution can be estimated with the dimethylglyoxime reagent. The correct statement (s) about the reaction and the product is (are)
in ammoniacal solution Ni2+ salts give cherry red precipitate of nickel (II) dimethylglyoximate
two dimethylglyoxiamte units are bound to one Ni2+
in the complex dimethylglyoximate units are hydrogen bonded to each other
each dimethylglyoximate unit forms a six membered chelate ring wilth Ni2+
Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - MTG-WBJEECHAPTER - COORDINATION COMPOUNDSEXERCISE - WB JEE Previous Years Questions (Category 1: Single Option Correct Type)
6 Videos
- CHEMISTRY OF NON-METALLIC ELEMENTS AND THEIR COMPOUNDSBOOK - MTG-WBJEECHAPTER - CHEMISTRY OF NON-METALLIC ELEMENTS AND THEIR COMPOUNDSEXERCISE - WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 3 : One or More Option Correct Type)
1 Video
- ENVIRONMENTAL CHEMISTRY BOOK - MTG-WBJEECHAPTER - ENVIRONMENTAL CHEMISTRY EXERCISE - WB JEE Previous Years Questions ( CATEGORY 1 : Single Option Correct Type )
2 Videos
Similar Questions
Amongst [NiCl4]2−,[Ni(H2O)6]2+,[Ni(PPh3)2Cl2],[Ni(CO)4] and [Ni(CNO4)]2− the paramagnetic species are
View Solutionwhite phosphorus P4 has the following characteristics.
View SolutionAmong the followings , the one which is not a " greenhouse gas " , is
View SolutionDimethylglyoxime is coordinated to Ni2+ through .
View SolutionWhich of the statements is/are correct about the following reactions ? overset (H_(2)//Ni) rarr overset (H_(2)//Ni) rarr
View SolutionIn basic medium the amount of Ni2+ in a solution can be estimated with the dimethylglyoxime reagent. The correct statement (s) abot the reaction and the product is ( are )
View SolutionWhen dimethylglyoxime is added to Ni2+, which of the following statements or observations is wrong ?
View SolutionWhich reagent can be used to estimate Ni2+ ions ?
View SolutionIn basic medium the amount of Ni2+ in a solution can be estimated with dimenthylglyoxime regent. The correct statement(s) about the reaction and the product is (are) -
View SolutionDimethylglyoxime is used for estimation of
View Solutionক্ষারীয় দ্রবণে Ni2+ পরিমাপ করা যায় ডাইমিথাইলগ্লাইঅক্সিম বিকারকের সাহায্যে। বিক্রিয়া ও উৎপন্ন যৌগ সম্পর্কে সঠিক বিবৃতি (গুলি) হল-
View SolutionIn basic medium the amount of Ni2+ in a solution can be estimated with the dimethylglyoxime reagent. The correct statemnt(s) about the reaction an the product is (are)
View Solutionक्षारीय माध्यम में एक विलयन में Ni2+ की मात्रा डाईमिथाइल ग्लायऑक्जिम अभिकर्मक के साथ निरूपित कर सकते हैं। इस अभिक्रिया तथा उत्पाद के बारे में सही कथन है/हैं
View Solutionক্ষারীয় দ্রবণে Ni2+ এর পরিমাপ করা যায় ডাইমিথাইল গ্লাইঅক্সিম বিকারকের সাহায্যে। সঠিক বিবৃতি(গুলি), যা বিক্রিয়া এবং উৎপন্ন যৌগের ক্ষেত্রে প্রযােজ্য, হল
View SolutionRed precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammoniacal Ni(II). Which of the following statements is not true ?
View Solution