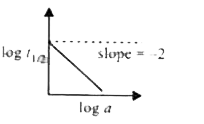
A graph between log t1/2 and log a (abscissa), a being the initial concentration of A in the reaction A → product, is depicted in the figure. The rate law is
−d[A]dt=k
−d[A]dt=k[A]
−d[A]dt=k[A]2
−d[A]dt=k[A]3
The correct Answer is:d
t1/2=ka1−n, n being order, k=rate constant log t1/2=log k+(1-n)log a
Slope, (1-n) = -2, n = 3
Topper's Solved these Questions
- CHEMICAL DYNAMICS Book:MTG-WBJEEChapter:CHEMICAL DYNAMICS Exercise:WB JEE Previous Years Questions (CATEGORY 1 : Single Option Correct Type )(1 Mark)
Explore 4 Videos
- CHEMICAL DYNAMICS Book:MTG-WBJEEChapter:CHEMICAL DYNAMICS Exercise:WB JEE Previous Years Questions (CATEGORY 2: Single Option Correct Type )(2 Marks)
Explore 3 Videos
- CHEMICAL DYNAMICS Book:MTG-WBJEEChapter:CHEMICAL DYNAMICS Exercise:WB JEE Previous Years Questions (CATEGORY 3 : One or More than One Option Correct Type)(2 Marks)
Explore 2 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:MTG-WBJEEChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:WB JEE Previous Years Questons (Category 2 : One or More than one Option Correct Type )
Explore 3 Videos
- CHEMICAL ENERGETICSBook:MTG-WBJEEChapter:CHEMICAL ENERGETICSExercise:WB JEE PREVIOUS YEARS QUESTIONS (ONE OR MORE THAN ONE OPTION CORRECT TYPE )
Explore 2 Videos
Similar Questions
A graph between logt12 and log a (abscissa), a being the initial concentration of A in the reaction For reaction A→Product, the rate law is :
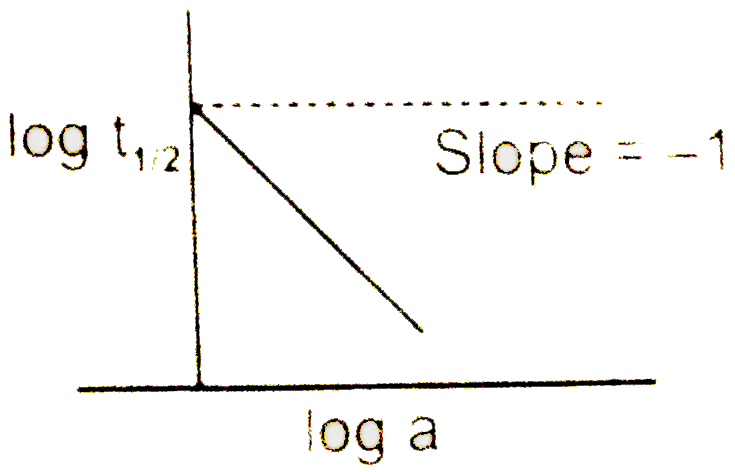
View SolutionA graph between logt12 and log a (abscissa), a being the initial concentration of A in the reaction For reaction A→Product, the rate law is :

View Solution
Knowledge Check
A graph between logt12 and log a (abscissa), a being the initial concentration of A in the reaction For reaction A→Product, the rate law is :
 A−d[A]dt=KB−d[A]dt=K[A]C−d[A]dt=K[A]2D−d[A]dt=K[A]3
A−d[A]dt=KB−d[A]dt=K[A]C−d[A]dt=K[A]2D−d[A]dt=K[A]3A graph between logt12 and log a (abscissa), a being the initial concentration of A in the reaction For reaction A→Product, the rate law is :
 A−d[A]dt=kB−d[A]dt=k[A]C−d[A]dt=k[A]2D−d[A]dt=k[A]3
A−d[A]dt=kB−d[A]dt=k[A]C−d[A]dt=k[A]2D−d[A]dt=k[A]3Following is the graph between logT50 and loga (a= initial concentration) for a given reaction at 27∘C. Hence order is
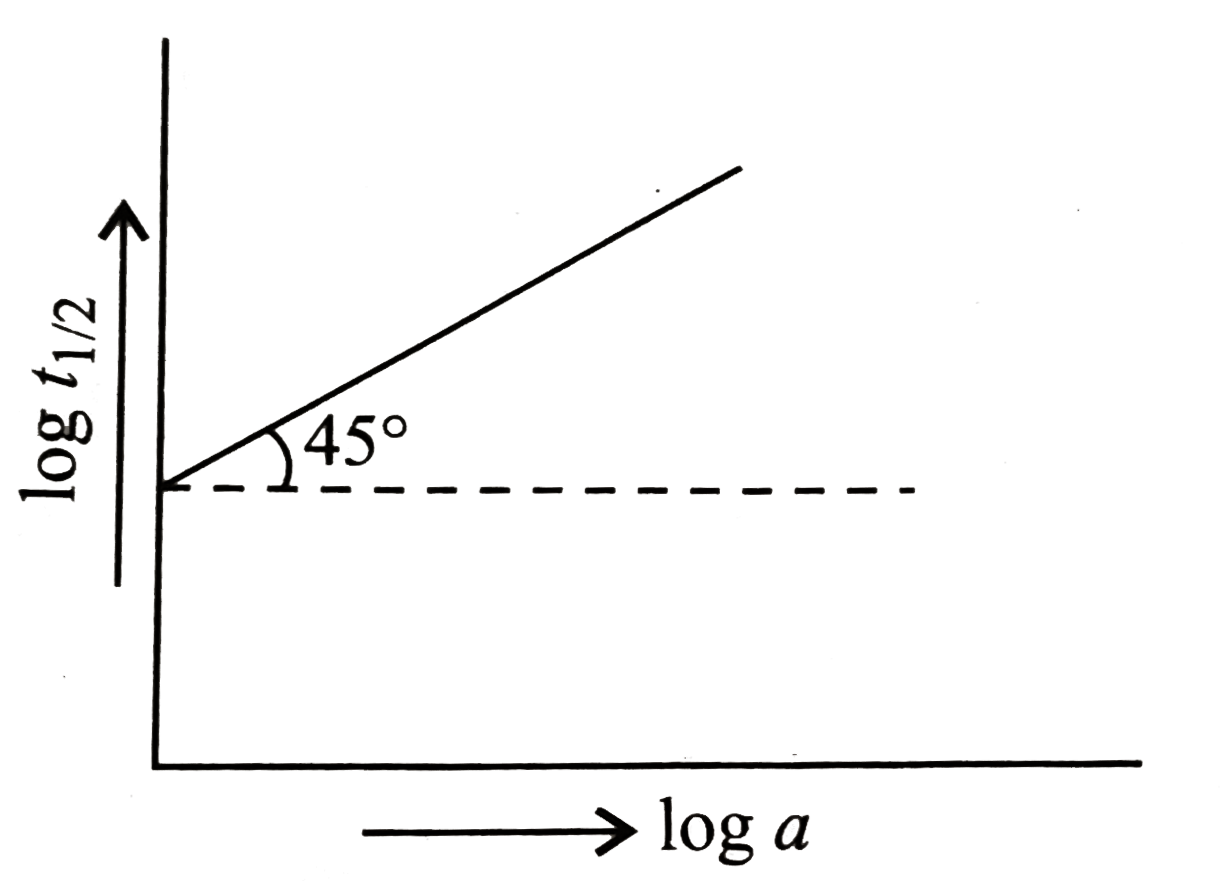 A0B1C2D3
A0B1C2D3
Following is the graph between logt1/2 and loga (a initial concentration) for a given reaction at 300K

Find the order of reaction.
View SolutionFollowing is the graph between logT50 and loga (a= initial concentration) for a given reaction at 27∘C. Hence order is

View SolutionFollowing is the graph between logt1/2 and loga (a initial concentration) for a given reaction at 27∘C.

Find the order of reaction.
View SolutionThe graph between log t1/2 and log a at a given temperature is
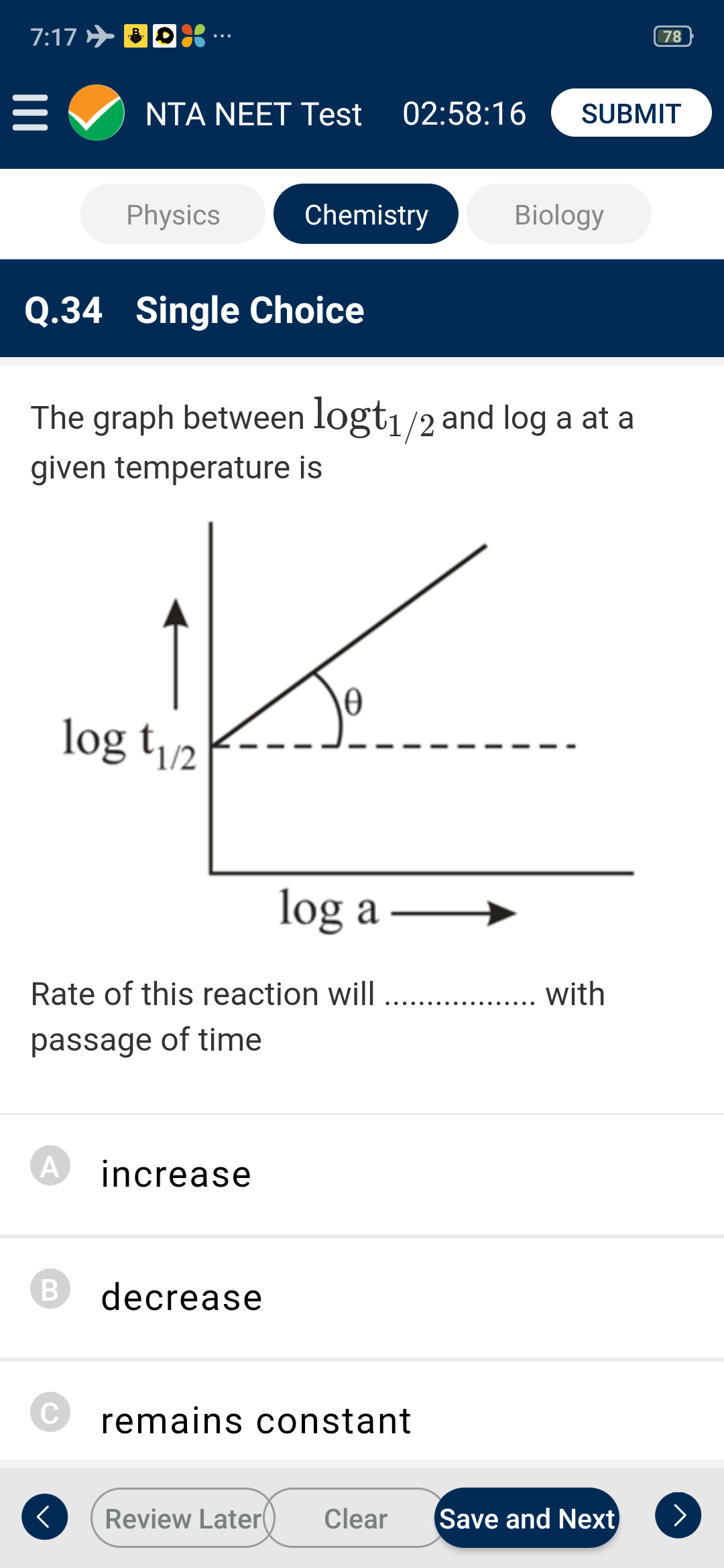
Rate of this reaction will …….with passage of time
View SolutionFollowing is the graph between log T50 and log a (a = initial concentration) for a given reaction at 27∘C.
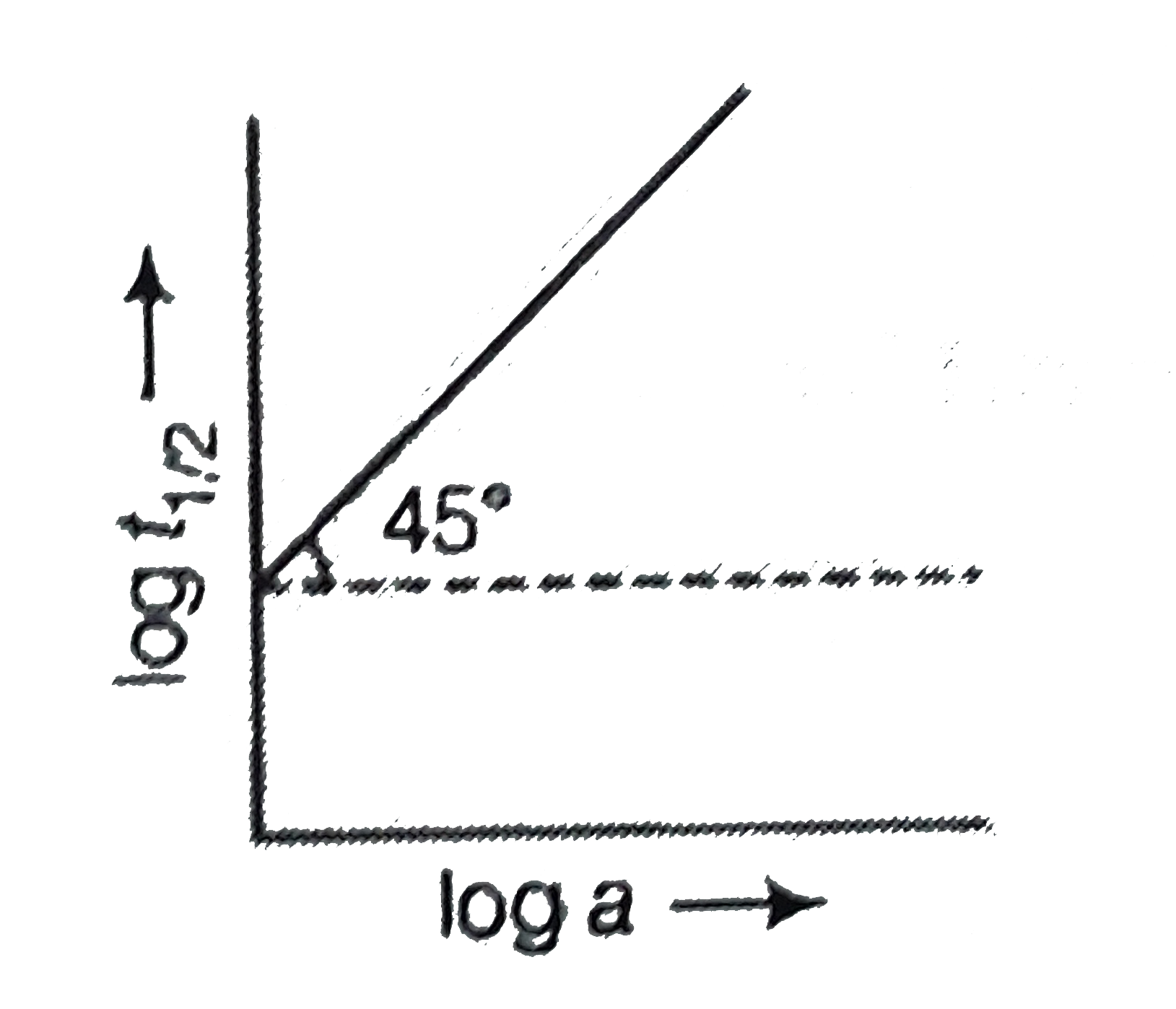
Hence, order is
View Solution

