Which of the following are correct statements?
A
When mixture is more volatile, there is positive deviation from Raoult's law.
B
When mixture is less volatile, there is negative deviation froin Raoult's law
C
Ethanol and water form ideal solution.
D
CHCl3 and water form ideal solution
Text Solution
The correct Answer is:A, B
(a,b)
|
Similar Questions
Knowledge Check
Which of the following are correct statement regarding these molecules.
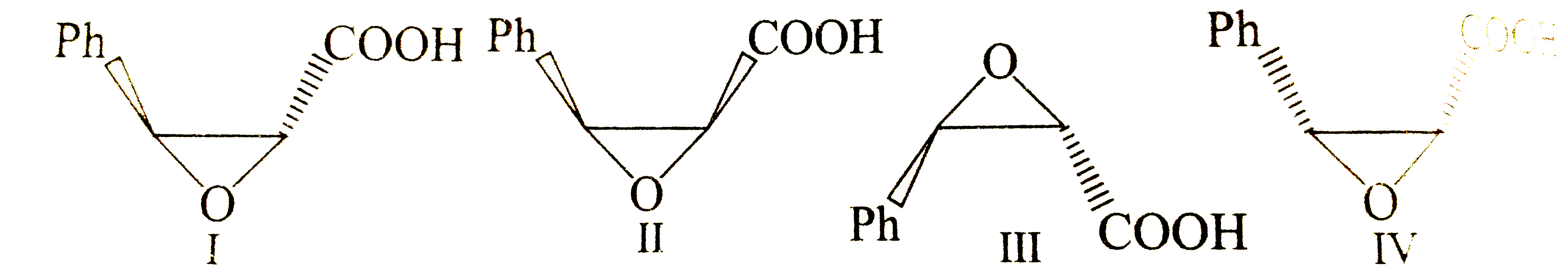 ACompound I and II are diastereomersBCompound II and IV are enantiomersCCompound I and III are enantimersDCompound III and IV are homomers
ACompound I and II are diastereomersBCompound II and IV are enantiomersCCompound I and III are enantimersDCompound III and IV are homomersWhich of the following are correct statement regarding these molecules.
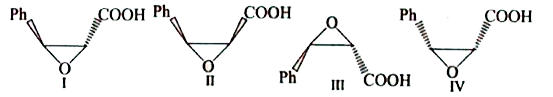 ACompound I and II are diastereomersBCompound II and IV are enantiomersCCompound I and III are enantiomersDCompound III and IV are homomers
ACompound I and II are diastereomersBCompound II and IV are enantiomersCCompound I and III are enantiomersDCompound III and IV are homomersWhich of the following is correct statement?
AF2 has higher dissociation energy than Cl2.BF has higher electron affinity than Cl.CHF is stronger acid than HCl.DBoiling point increases down the group in halogens.

