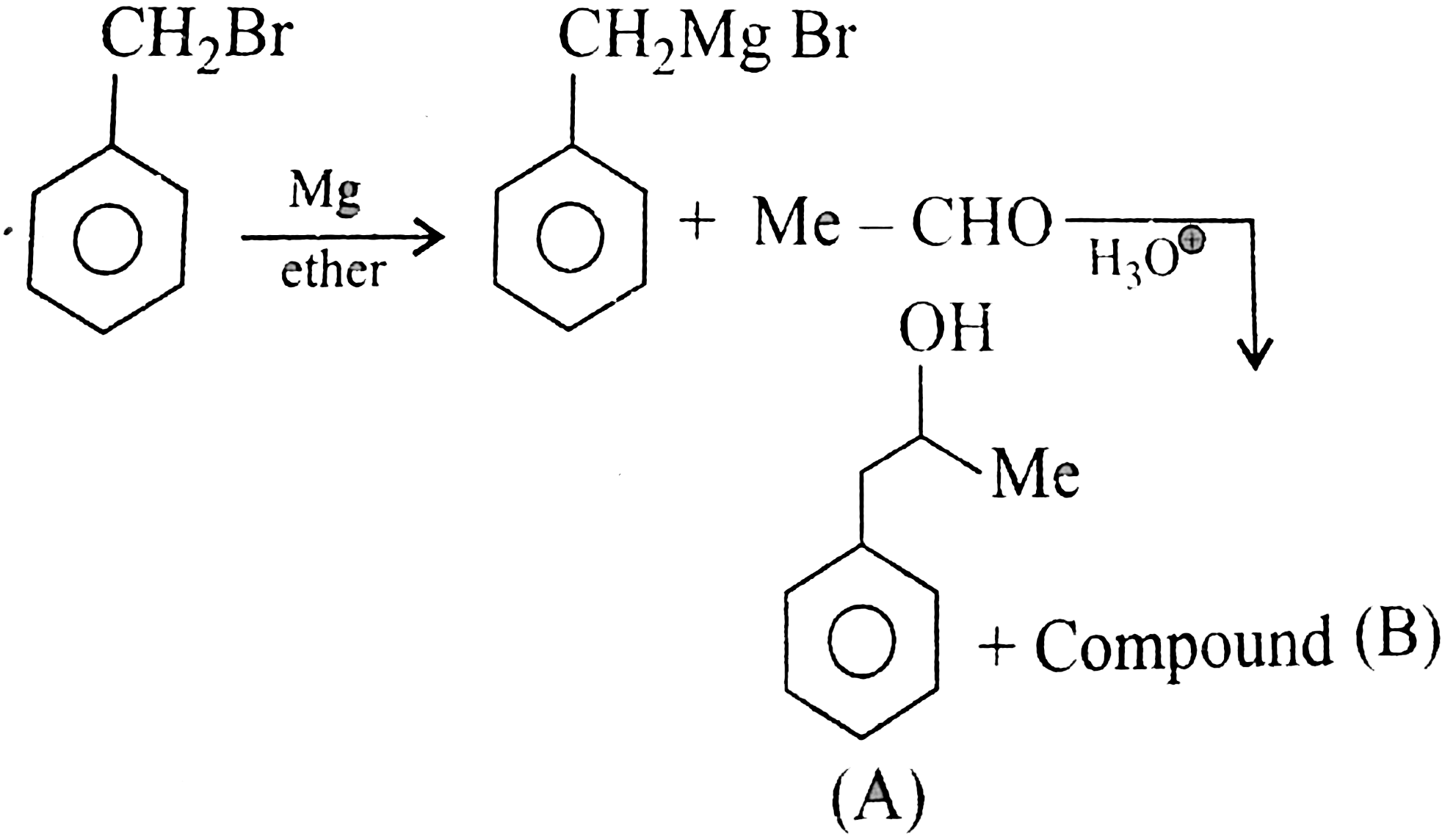
Compound (B) is an ismoer of (A0. Compound (B) shows positive iodoform test and gives o− toule acid. What is (B) ? Explain its formation.
Video Solution
Text Solution
(B) is an isomer of (A) and shows iodofrom reaction.threrfore, the side chain must contain either
⎛⎜
⎜⎝O∣∣−C−CH3⎞⎟
⎟⎠ or ⎛⎜
⎜⎝−CH∣OH−CH3⎞⎟
⎟⎠ group.
Compound (B) may be:
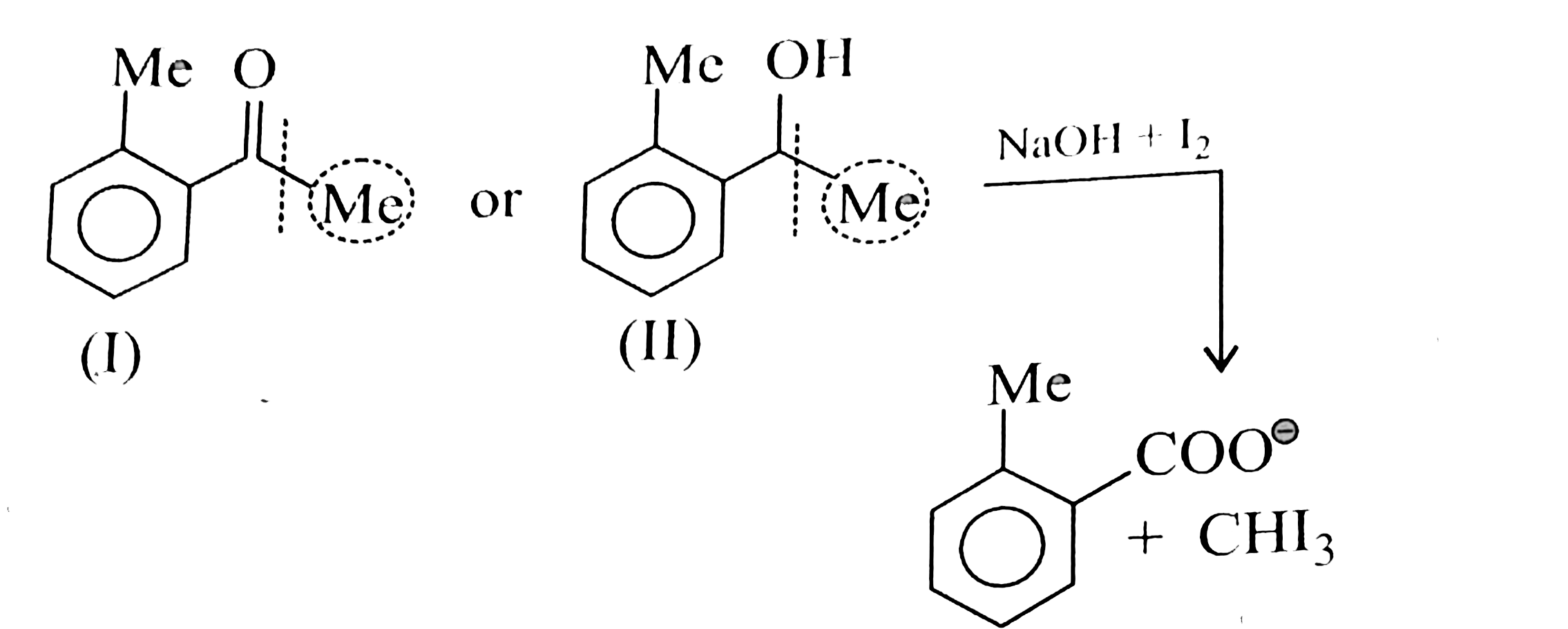
But (I) is not an isomer of (A) (molecular formula C6H10O), (II) is an isomer of (A). Hence, compound (B) is (II).
Formaion of (II): It is involes the rearrangement . The (CH3CHO) group is attached to o− positon of the ring. due to the plarisation of
 in which o-position of the ring behaves as the nucleophilic center. <br> <img alt=)
|
Similar Questions
Knowledge Check
A compound that shows positive iodoform test is
A2 - pentanoneB3 - pentanoneC3 - pentanolD1 - pentanolA compound that gives a positive iodoform test is
Apentan-1-olBpentane-3-oneCpentane-2-oneDpentanalA compound that gives a positive iodoform test is:
Aa.1- PentanolBb.3-PentanoneCc.2- PentanalDd.
The compound which gives positive iodoform test is :
View SolutionCompound (A) undergoes Cannizzaro reaction and (B) undergeos positive iodoform test. Therefore
View SolutionWhich of the following compound will give positive iodoform test ?
View SolutionSelect the compound which will give a positive iodoform test.
View SolutionWhich one of the following compounds gives a positive iodoform test?
View Solution
