In each of the following reactions, a new ring is formed. Use curved arrow notation to explain the formation of the new ring.
a. 1,4− Dibromobutance (0.1mol) is treated with sodiuk suplhile (0.1mol) in aqueous ethabnol.
b. 1− Choloro −2 (2− hyddroxyphenyl) ethane is treated with aquencous sodium hydroxide.
c. o− Methoxybenyl bromide reacts faster than p− nitrobenyzl bromide with ethanol to form an ether product. Explain why.
d. Explain the formation of the two products in the following reactions:
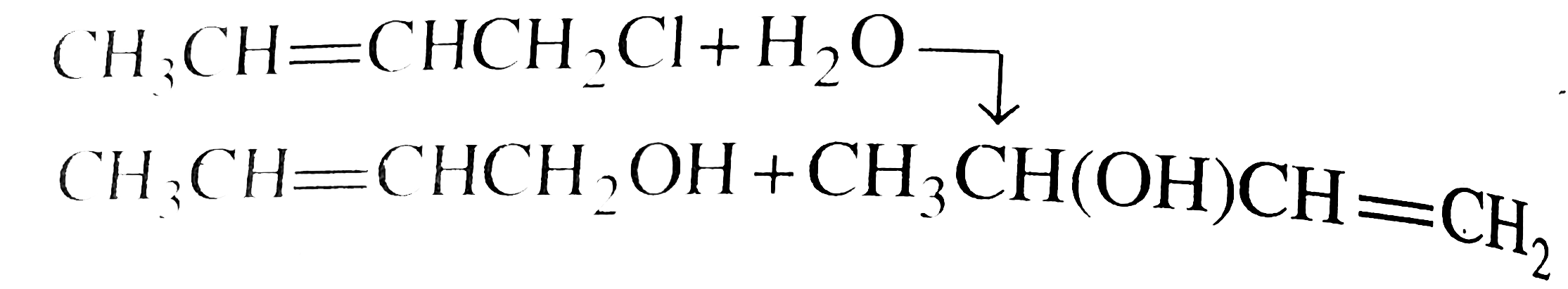
e. What mass of propene obtained from 34.0gm of I− iodopropane on heating with ethanolic KOH, if the yield is 36% ?
f. The nucleophilic substitution of primary alkyl chlorides with sodium acated acetate is catalysed by sodium iodide. Explain why.

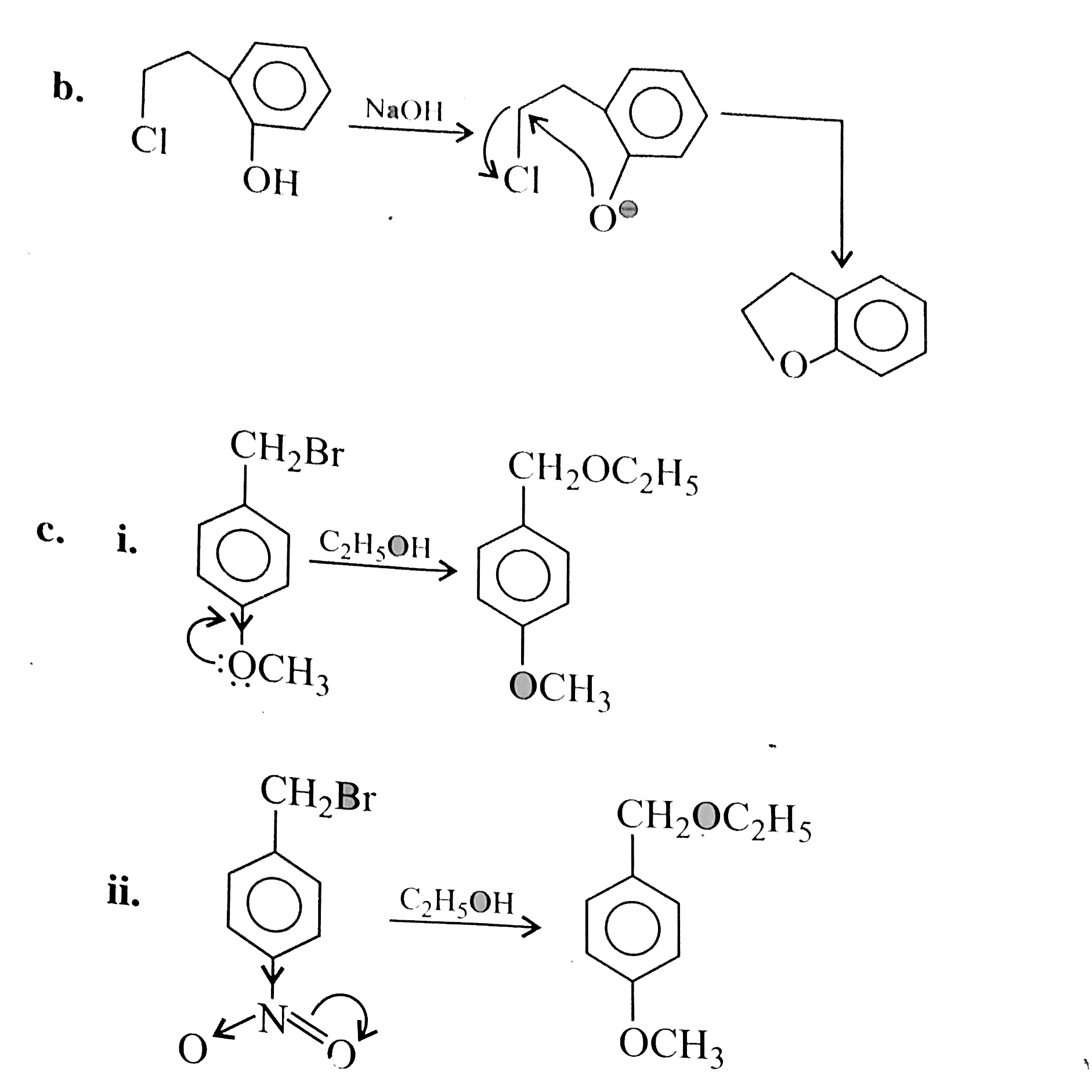
The electron-dontaing group (−OCH3) by resonance increases the stabiliy of benzyl of benyl carbonium ion, whereas the electron-withdrawing group [(NO2) group] by resonance decreases the stablity of benzyl carbonium ion. Hence, reaction (1) is faster than (2) with ethanol to give ether by SN−1 mechanism.
d. 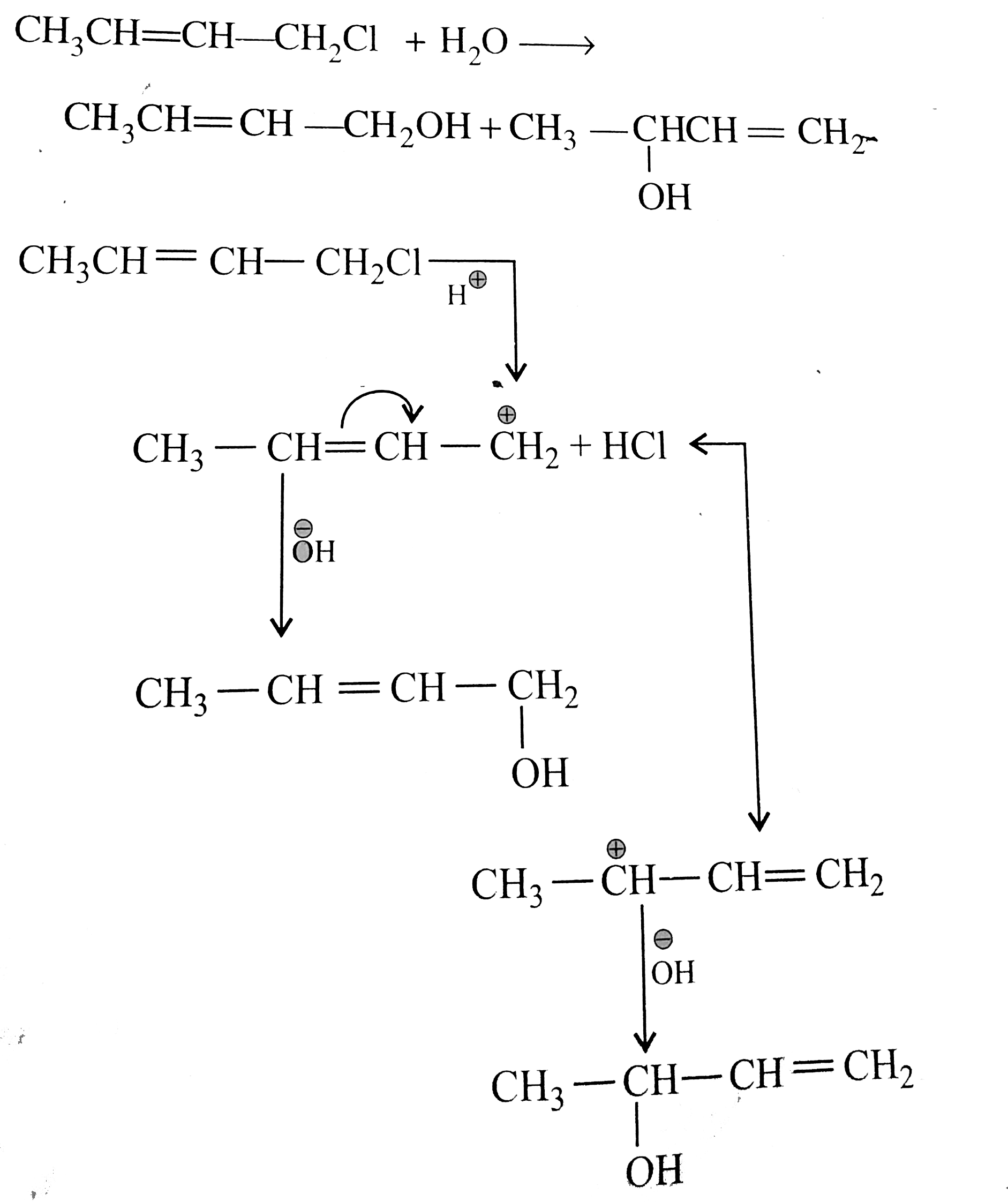
Two products are formed due to allylic rearrangements.
e. CH3CH2CH2IEthanolic−−−−−−−→KOHCH3CH=CH2
Mw=170gm,Mw=42gm
170gm of iodopropane gives 42gm of propene.
34gm of iodopropane gives 42×34170=8.4gm
If the yield is 36% then Wt. of propene =8.4×34100
=3.024gm
f. R−Cl+CH3−COONa→R−O−O∣∣C−CH3+NaCl
This reaction is SN2. Nuclophile is CH3COO∘
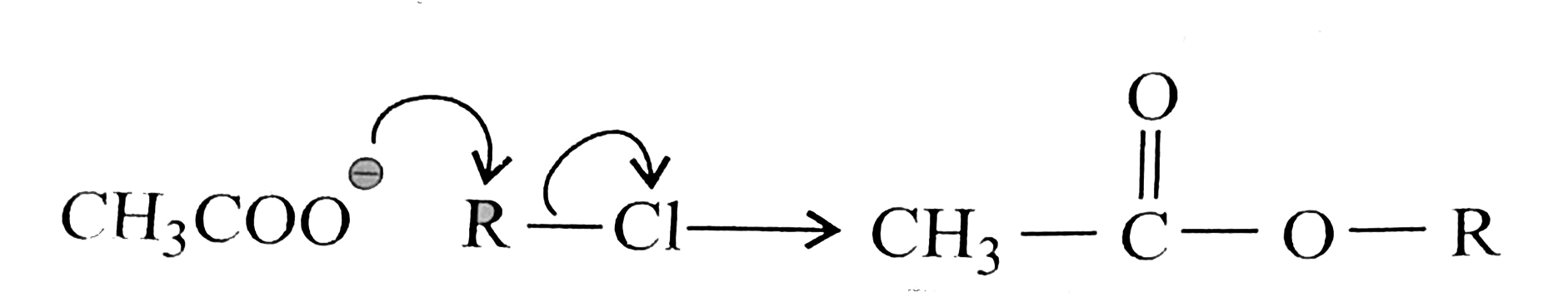 But in alkyl chloride, (C−Cl) is a strong bond and is difficult to break. But in the presence of Nal,(R−Cl) is converted to (R−I) (Finkelstein reaction) and therefore, in (R−1) the (C−1) bond is weak, which can e easily cleaved. So SN2 reaction is cataysed in the presence of Nal.
But in alkyl chloride, (C−Cl) is a strong bond and is difficult to break. But in the presence of Nal,(R−Cl) is converted to (R−I) (Finkelstein reaction) and therefore, in (R−1) the (C−1) bond is weak, which can e easily cleaved. So SN2 reaction is cataysed in the presence of Nal.
R−Cl+Nal→R−I+NaCl
Topper's Solved these Questions
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES BOOK - CENGAGE CHEMISTRYCHAPTER - AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES EXERCISE - Exercises Concept Application
4 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES BOOK - CENGAGE CHEMISTRYCHAPTER - AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES EXERCISE - Exercises Linked Compreshension Type
11 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES BOOK - CENGAGE CHEMISTRYCHAPTER - AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES EXERCISE - Exercises Subjective
17 Videos
- APPENDIX INORGANIC VOLUME 2BOOK - CENGAGE CHEMISTRYCHAPTER - APPENDIX INORGANIC VOLUME 2EXERCISE - Short Answer Type
179 Videos
- BIOMOLECULESBOOK - CENGAGE CHEMISTRYCHAPTER - BIOMOLECULESEXERCISE - Exercises Archives (Analytical And Descriptive)
8 Videos
Similar Questions
How will you bring about the following conversations in not more than two steps?
a. Ethanlol to but −1 yne
b. Ethnee to bromoethene
c. Propene to 1− Nitropropane
d. Toluene to benzyl alchol
e. Propane to propyne
f. Ethanol to ethyl fluoride
g. Bromomethane to prepanone
h. But −1−e≠ to but −2−e≠
i. 1− Chlorobutane to n− octane
j. Bromocethane to cis-hex −3− ene
k. Benyl alchol to phenylethanenitrile
l. Benzene to biphenyl
m. Cyclopentene to cycolpentya −1,3− diene
n. Anline to phenyl isocyanide
View Solution
The type of stereosim arising from restricted rotation about a single bond and where the stereoisomers can be isolated is called.
View SolutionGive the chemical test to distinguish between
(a) Hexane (I), MeCH=CHCl(II),
Propylchloride (III), and CH2=CHCH2Cl(IV)
(b) (V)CH3Cl, (VI) CH_(3)Br,and(VII) CH_(3)I
View SolutionWhich metals are generally extracted by the electrolytic processes ? What positions these metals generally occupy in the periodic table?
View SolutionWrite the structure of alanine at pH=2andpH=10.
View SolutionWhen sodium salt of ethanol is treated with ethyl bromide, the product formed is :
View SolutionReaction of methyl bromide with aqueous sodium hydroxide involves
View SolutionEthyl bromide , methyl bromide, and sodium will react to form
View Solutionनिम्नलिखित अभिक्रियाओं मे से प्रत्येक मे एक नई रिंग का निर्माण होता है । नई रिंग के निर्माण की व्याख्या करने के लिए मुड़े हुए तीर के संकेत का उपयोग कीजिए -
(a) 1, 4-डाइब्रोमोब्यूटेन (0.1 मोल) की क्रिया जलीय ऐथेनॉल युक्त सोडियम सल्फाइड (0.1 मोल) के साथ कराई जाती है ।
(b) 1-क्लोरो-2-(2-हाइड्रॉक्सीफेनिल) ऐथेन जलीय सोडियम हाइड्राक्साइड के साथ क्रिया करता है ।
View Solutionp-नाइट्रोबैन्जिल की अपेक्षा p-मेथॉकसी बैन्जिल ब्रोमाइड ईथर निर्माण के लिए ऐथेनॉल से अधिक तेजी से क्रिया करता है । कारण स्पष्ट कीजिए ।
View Solution(a) The nucleophilic substitution of primary alkyl chlorides with sodium acetate is catalysed by sodium iodide. (b) p-Methoxybenzayl bromide reacts faster than p-nitrobenzyl bromide with ethanol to form a ether product.
View SolutionReaction of methyl bromide with aqueous sodium hydroxide involves
View Solutionn- प्रोपिल ब्रोमाइड, एथेनॉलिक पोटैशियम हाइड्रोक्साइड के साथ क्रिया करके बानाता है
View Solutionp-methoxybenzyl bromide reacts faster than p-nitrobenzyl bromide with ethanol to form an ether product. Explain why?
View SolutionReaction of methyl bromide with aqueous sodium hydroxide involves
View Solutionp-methoxylbenzyl bromide reacts faster than p-nitrobenzyl bromide with ethanol to form an ether product. Explain, why ?
View Solution

