SN reaction is given by these compounds, which have a nuclophilic group and a good leaving EWG. It should be stable after leaving with bonding pair of ¯e's and it should have high polarisability.
Nucliphilic aliphatic substituion reaction is mainly of two types SN−1 and SN2.SN−1 mechanism is a two step process. Reaction velocity of SN−1 depends only on the concentration of the subtrate. It proceeds via the formation of carbocation, optically active substrate gives (⊕) and (o−) froms of the product.
In most of the cases, the product usually consists of 5−20% inverted and (95−80%) racemised species. The more stable is the carbocation, the greater is the propotion of racemission. In solvolysis reaction, the more nucleophillic is the solvent, the greater is the proportion of inversion.
Which of the following gives SN1 reaction?
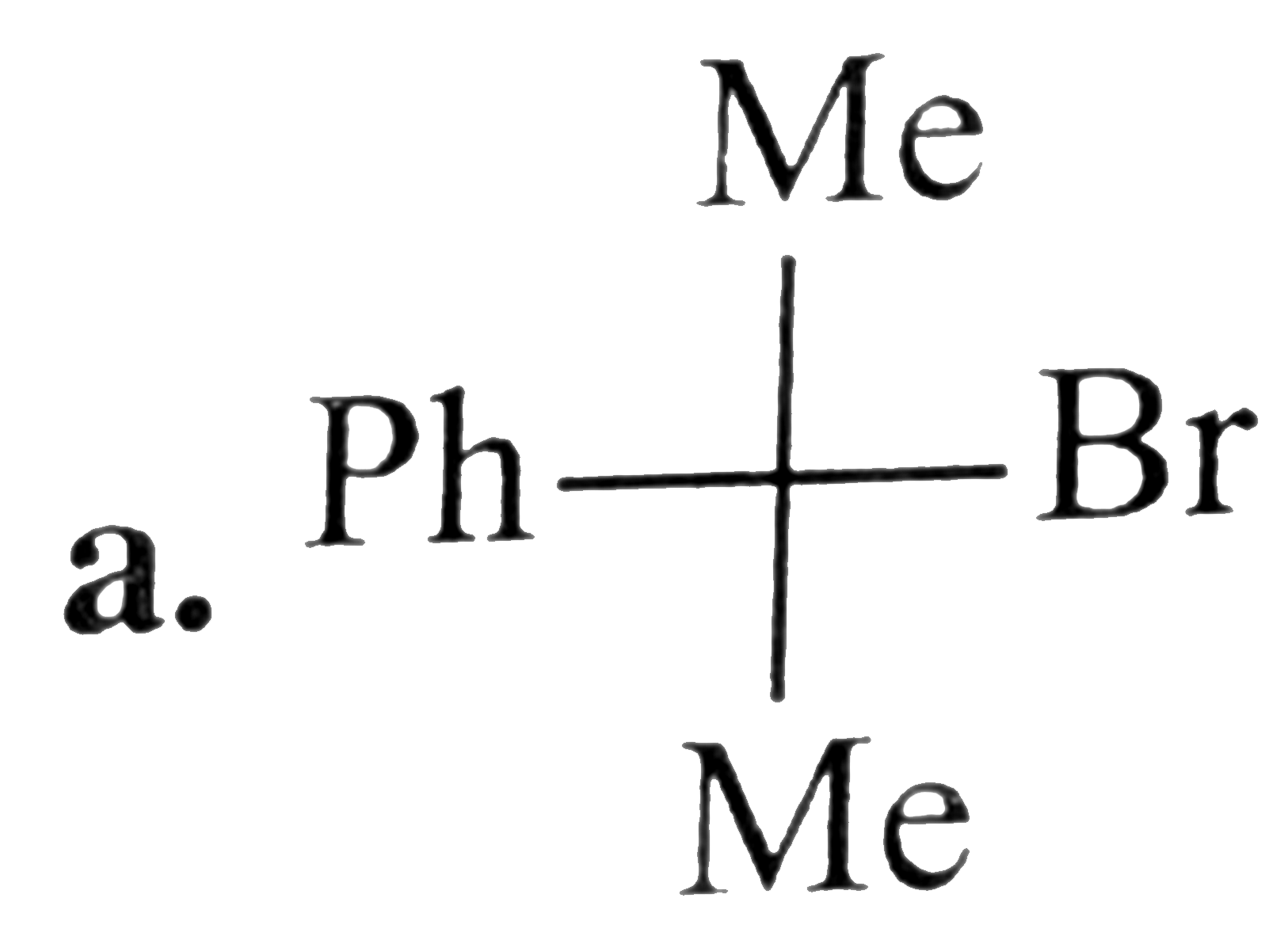

MeBr
All
The correct Answer is:a
It is 3∘RX and forms stable PhMe2C⊕ ion.
Similar Questions
SN reaction is given by these compounds, which have a nuclophilic group and a good leaving EWG. It should be stable after leaving with bonding pair of ¯e's and it should have high polarisability.
Nucliphilic aliphatic substituion reaction is mainly of two types SN−1 and SN2.SN−1 mechanism is a two step process. Reaction velocity of SN−1 depends only on the concentration of the subtrate. It proceeds via the formation of carbocation, optically active substrate gives (⊕) and (o−) froms of the product.
In most of the cases, the product usually consists of 5−20% inverted and (95−80%) racemised species. The more stable is the carbocation, the greater is the propotion of racemission. In solvolysis reaction, the more nucleophillic is the solvent, the greater is the proportion of inversion.
Which of the following will give SN reaction?
View SolutionSN reaction is given by these compounds, which have a nuclophilic group and a good leaving EWG. It should be stable after leaving with bonding pair of ¯e's and it should have high polarisability.
Nucliphilic aliphatic substituion reaction is mainly of two types SN−1 and SN2.SN−1 mechanism is a two step process. Reaction velocity of SN−1 depends only on the concentration of the subtrate. It proceeds via the formation of carbocation, optically active substrate gives (⊕) and (o−) froms of the product.
In most of the cases, the product usually consists of 5−20% inverted and (95−80%) racemised species. The more stable is the carbocation, the greater is the propotion of racemisation. In solvolysis reaction, the more nucleophillic is the solvent, the greater is the proportion of inversion.
For the reaction
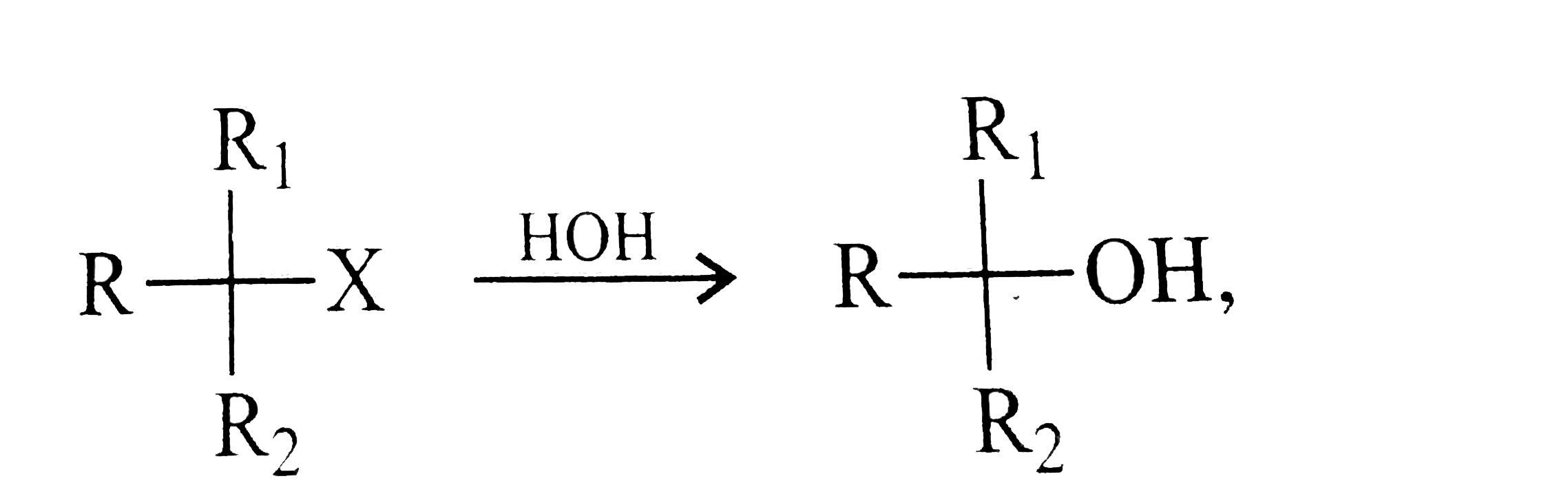
which substrate will give maximum racemismision ?
View Solution
Knowledge Check
SN reaction is given by these compounds, which have a nuclophilic group and a good leaving EWG. It should be stable after leaving with bonding pair of ¯e's and it should have high polarisability.
Nucliphilic aliphatic substituion reaction is mainly of two types SN−1 and SN2.SN−1 mechanism is a two step process. Reaction velocity of SN−1 depends only on the concentration of the subtrate. It proceeds via the formation of carbocation, optically active substrate gives (⊕) and (o−) froms of the product.
In most of the cases, the product usually consists of 5−20% inverted and (95−80%) racemised species. The more stable is the carbocation, the greater is the propotion of racemission. In solvolysis reaction, the more nucleophillic is the solvent, the greater is the proportion of inversion.
Which of the following will give SN reaction?AR−BrBR−N3CR−O⊕H2DAllSN reaction is given by these compounds, which have a nuclophilic group and a good leaving EWG. It should be stable after leaving with bonding pair of ¯e's and it should have high polarisability.
Nucliphilic aliphatic substituion reaction is mainly of two types SN−1 and SN2.SN−1 mechanism is a two step process. Reaction velocity of SN−1 depends only on the concentration of the subtrate. It proceeds via the formation of carbocation, optically active substrate gives (⊕) and (o−) froms of the product.
In most of the cases, the product usually consists of 5−20% inverted and (95−80%) racemised species. The more stable is the carbocation, the greater is the propotion of racemission. In solvolysis reaction, the more nucleophillic is the solvent, the greater is the proportion of inversion.
Fugacity power of which group will be maximum?ACH3−O−O∣∣S∣∣O−C2H5BCH3−O−O∣∣S∣∣O−CH3C DCH3−O−O∣∣C−CH3
DCH3−O−O∣∣C−CH3SN1 reaction is.
Asingle step reationBa reaction involving free readical intermediateCtwo step reactionDtwo step and reaction intermediate is carbocation
SN1 and SN2 mechanisms
View SolutionSN1 and SN2 reaction
View SolutionPredicrt the order of reactivity of the following compounds in SN1 and SN2 reactions.
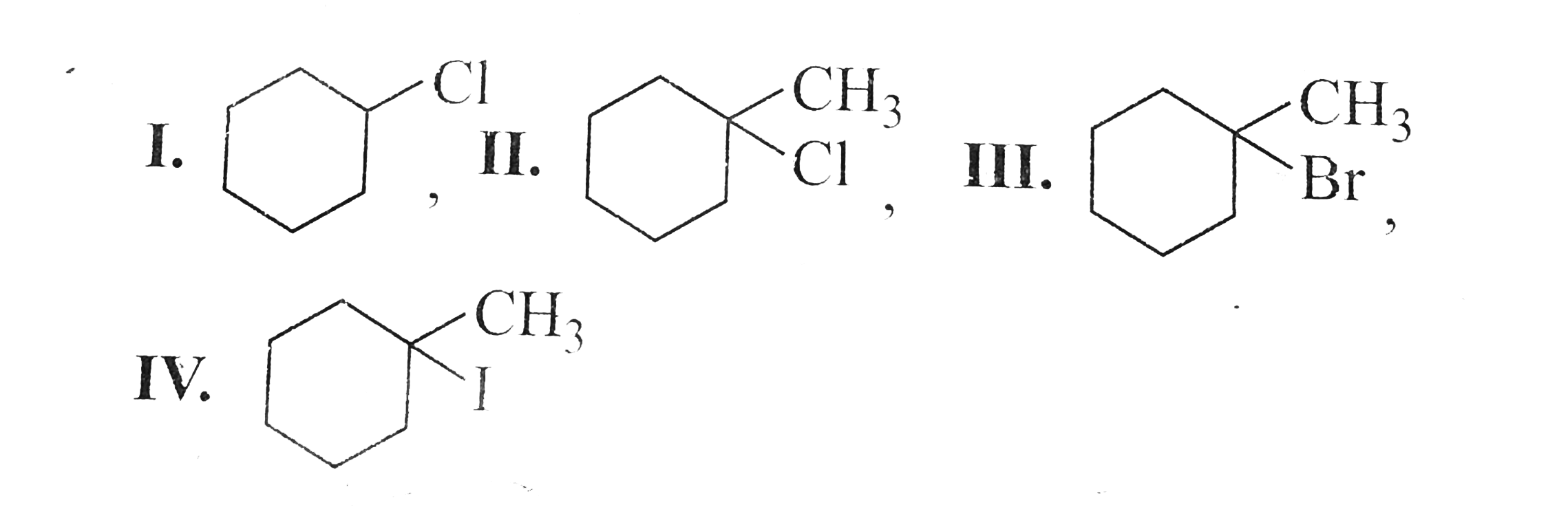
View SolutionPredicrt the order of reactivity of the following compounds in SN1 and SN2 reactions.

View SolutionS1N reaction is favoured by
View Solution

