Which is the most effective ion in an SN2 displacement on methyl bromide?
A
C6H5Oθ
B
overset(theta)(O)H
C
C6H5Oθ
D
CH3COOθ
Text Solution
The correct Answer is:a
More the nucelophilc character more the SN2 reaction. Basically and nucleophilicity are same when the nucelphilic centres are same.
Acidic order:
HNO3>CH3CCOOH>C6H5OH>H2O>C2H5OH
Basic and nucleophiliic order:
NOo−3<CHCOOo−<C6H5OOo−<Oo−H<C2H5o−O
|
Similar Questions
Knowledge Check
Which does not give methyl bromide?
ACH3OH+HBrBCH3OH+Br2CCH3OH+PBr3DCH3COOAg+Br2Most effective ion to coagulate a negative sol is:
APO3−4BAl3+CBa2+DK+Most effective ion to coagulate a negative sol is :
APO3−4BAl3+CBa2+DK+
Which of the following halides will be most reactive in SN2 reaction and SN2 reaction respectively?
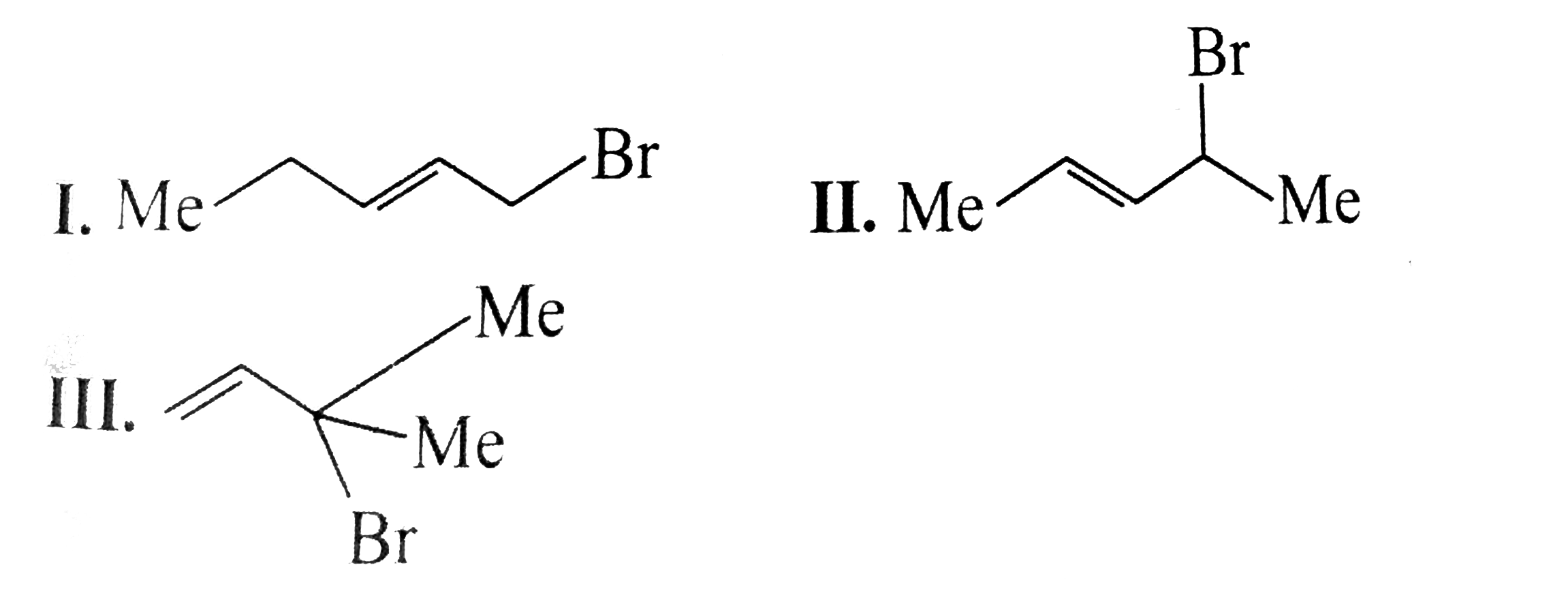
View SolutionWhich of the following ions is most effective in the coagulation of ferric hydroxide solution?
View SolutionMost effective ion to coagulate a negative sol is :
View SolutionWhich of the following ions is most effective in the coagulation of ferric hydroxide solution ?
View SolutionWhich of the following ions is most effective in the coagulation of ferric hydroxide solution?
View Solution

