Assertion (A): SN1 reaction is carried out in the presence of a plar protic solvent.
Reason (R): A polar protic solvent increases the stability of carbocation due to solvation.
Both (A) and (R) are true and (R0 is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true but (R) is false.
(A) is false but (R) is true.
The correct Answer is:d
NA
Topper's Solved these Questions
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Book:CENGAGE CHEMISTRYChapter:AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Exercise:Archives Single Correct
Explore 21 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Book:CENGAGE CHEMISTRYChapter:AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Exercise:Archives Single Correct Answer Type
Explore 3 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Book:CENGAGE CHEMISTRYChapter:AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Exercise:Exercises Assertion-Resoning Type
Explore 1 Video
- APPENDIX INORGANIC VOLUME 2Book:CENGAGE CHEMISTRYChapter:APPENDIX INORGANIC VOLUME 2Exercise:Short Answer Type
Explore 179 Videos
- BIOMOLECULESBook:CENGAGE CHEMISTRYChapter:BIOMOLECULESExercise:Exercises Archives (Analytical And Descriptive)
Explore 8 Videos
Similar Questions
Among the following compounds the one that is most reactive towards electrophilic nitration is
View Solution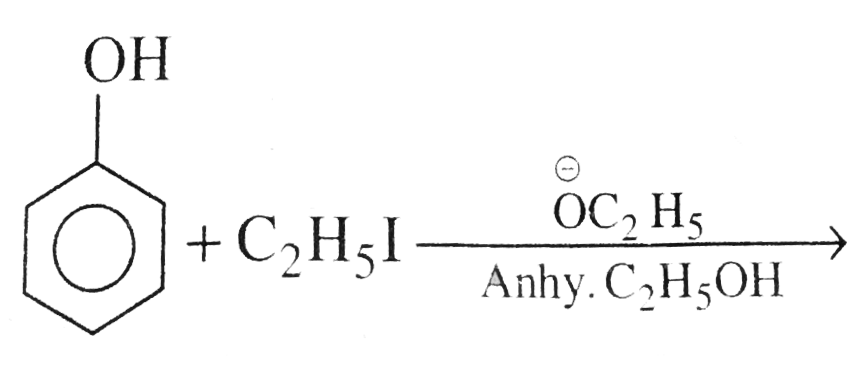
View SolutionAssertion (A):
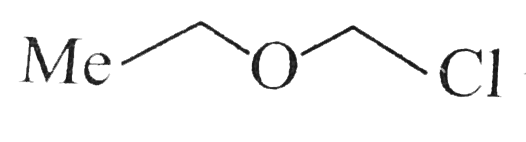 reacts faster with H2O than
reacts faster with H2O than 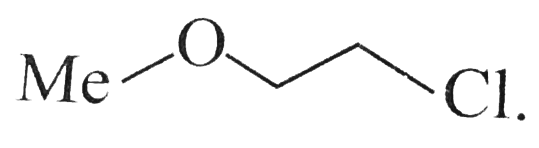
Reason (R): The carbocation of is more than the carbocation of
is more than the carbocation of 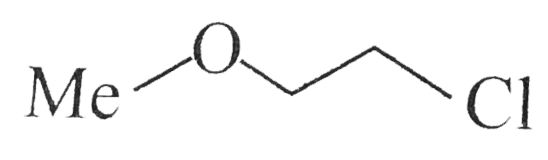
View SolutionWhich metals are generally extracted by the electrolytic processes ? What positions these metals generally occupy in the periodic table?
View SolutionWrite the structure of alanine at pH=2andpH=10.
View SolutionStatement =-1: Nucleophilicity order in polar -protic solvent is I^(-)lt Br^(-)lt ^(-)lt F^(-) Statement -2: Due to bigger zise of I^(-) it is less sovlated in polar a-protic solvent.
View SolutionAssertion (A) : SN^(2) reaction is carried out in the presence of polar aprotic solvent. Reason (R): Polar aprotic solvents do not contain acidic hydrogen.
View Solutionwhich is more reactive nucleophile in polar protic solvent ?
View SolutionWhy polar protic solvent are suitable for SN1 reaction?
View SolutionSTATEMENT-1 SN1 reaction is comied out in the presence of polar protic solvent and
STATEMENT-2 : Polar protic solvent increases stability of carboncation due to the solvation.
View SolutionIn the following questions, a statement of assertion (A) is following by a statement of reason (R ) A : In presence of DMSO Solvent, the rate of S_(N) 2 reaction inceases. R : DMSO is a polar protic solvent .
View Solutionप्रोटिक विलायक है
View SolutionWhich of the following are polar protic solvents
View SolutionStrongest nucleophile in polar-protic solvent is
View SolutionBest nucleophile in polar protic solvent is
View Solution
