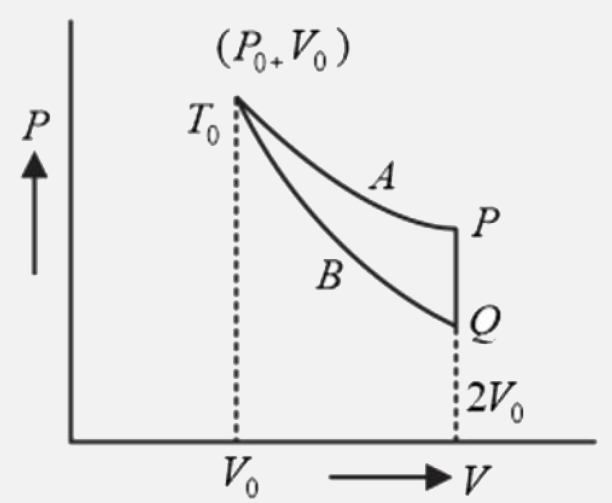
An ideal gas (1 mol, monatomic) is in the intial state P (see Fig.) on an isothermal A at temperature T0. It is brought under a constant volume (2V0) process to Q which lies on an adiabatic B intersecting the isothermal A at (P0,V0,T0). The change in the internal energy of the gas during the process is (in terms of T0)(22/3=1.587)

2.3T0
−4.6T0
−2.3T0
4.6T0
The correct Answer is:b
Temperature at state P=T0,since P lies on the isothermal of temperature T0 . If I be the temperature at Q. then for the adiabatic process B, we have
T0Vγ−10
⇒T=T02γ−1=T022/3[∴γ=53, so γ−1=23]
Change in the internal energy of the gas is
ΔU=VV(T−T0)=(Rγ−1)(T022/3−T0)
=3RT0(1−22/3)2×1.587=−0.55RT0=−4.6T0
Similar Questions
An ideal gas (1 mol ,monatomic) is in the initial state P (see diagram) on an isothermal curve A at a temperature T0 . It is brought under a constant volume (2V0) process to Q which lies on an adiabatic curve B intersecting the isothermal curve A at (P0,V0,T0) . The change in the internal energy of the gas (in terms of T0 ) during the process is (22/3=1.587)
View SolutionTwo moles of an ideal monatomic gas, initially at pressure p1 and volume V1, undergo an adiabatic compression until its volume is V2. Then the gas is given heat Q at constant volume V2.
(i) Sketch the complete process on a p-V diagram.
(b) Find the total work done by the gas, the total change in its internal energy and the final temperature of the gas. [Give your answer in terms of p1,V1,V2,QandR]
View Solution
Knowledge Check
One mole of hydrogen, assumed to be ideal, is adiabatically expanded from its initial state (P1,V1,T1) to the final state (P2,V2,T2). The decrease in the internal energy of the gas during this process will be given by
ACV(T1−T2)BCP(T1−T2)CCP+CV2(T1−T2)D(CP−CV)(T1−T2)One mole of monatomic gas is brought from state A to state B. The initial temperature at A is T0. The temperature at B will be –
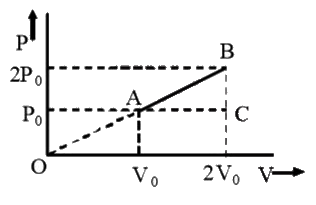 AT0B2T0C3T0D4T0
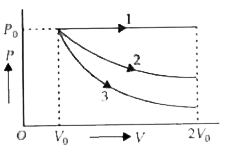
AT0B2T0C3T0D4T0A gas is expanded form volume V0→2V0 under three different processes as shown in the figure . Process 1 is isobaric process process 2 is isothermal and and process 3 is adiabatic .
Let ΔU1,ΔU2andΔU3 be the change in internal energy of the gs in these three processes then
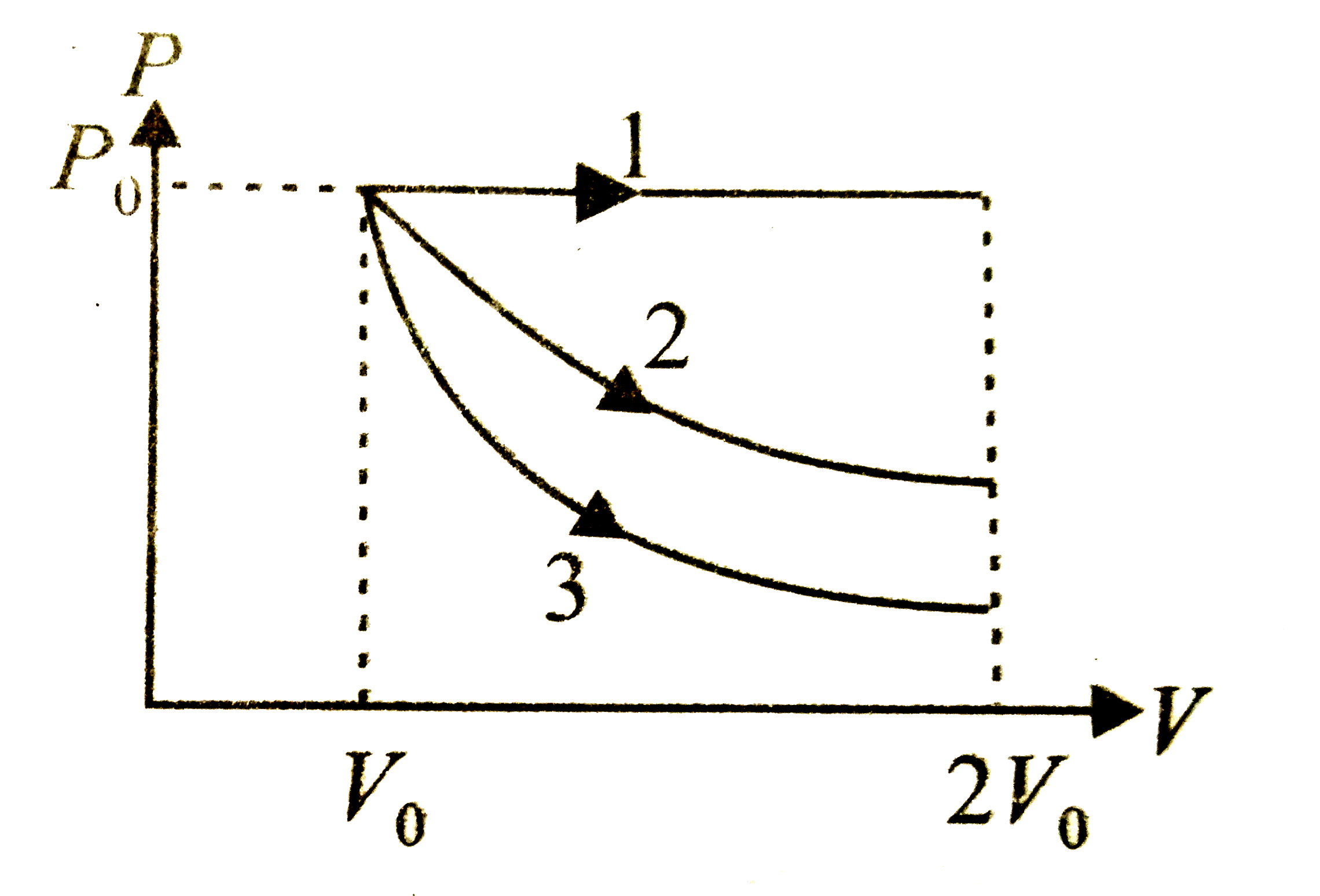 A△U1>△U2>△U3B△U1<△U3<△U2C△U2<△U3<△U3D△U2<△U3<△U1
A△U1>△U2>△U3B△U1<△U3<△U2C△U2<△U3<△U3D△U2<△U3<△U1
One mole of an ideal mono atomic gas is at a temperature T0=1000K and its pressure is P0. The gas is adiabatically cooled so that its pressure becomes 23P0. Thereafter, the gas is cooled at constant volume to reduce its pressure to P03 . Calculate the total heat absorbed by the gas during the process.
Take R=253Jmol−1K−1 and (23)2/5=0.85
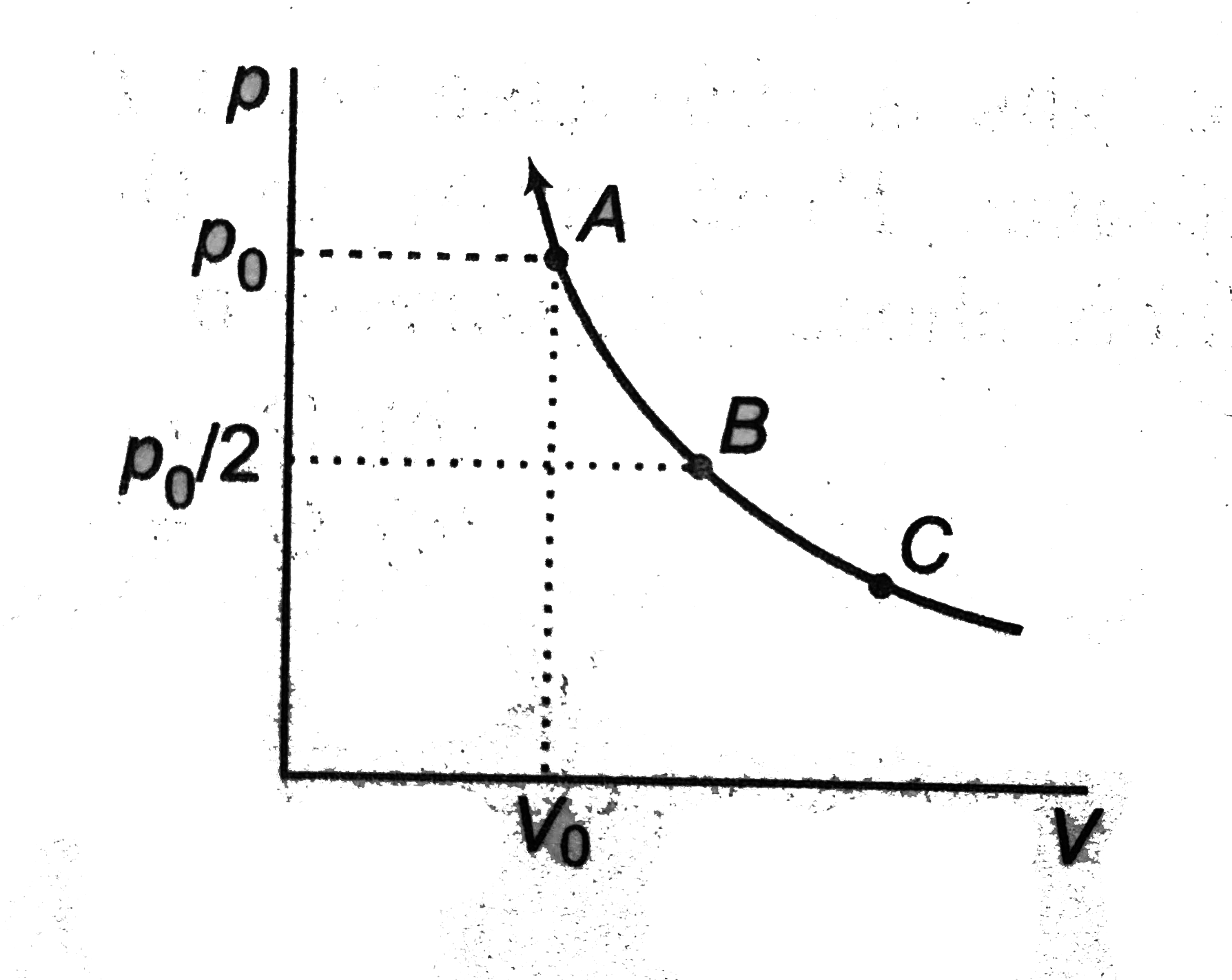
View SolutionThe state of an ideal gas is changed through an isothermal process at temperature T0 as shown in figure. The work done by the gas in going from state B to C is double the work done by gas in going from state A to B. If the pressure in the state B is p02, then the pressure of the gas in state C is
View SolutionA gas is expanded form volume V0→2V0 under three different processes as shown in the figure . Process 1 is isobaric process process 2 is isothermal and and process 3 is adiabatic .
Let ΔU1,ΔU2andΔU3 be the change in internal energy of the gs in these three processes then

View SolutionIn the following P-V diagram two adiabatics cut two isothermals at temperature T1andT2 (fig). The value of VaVd will be
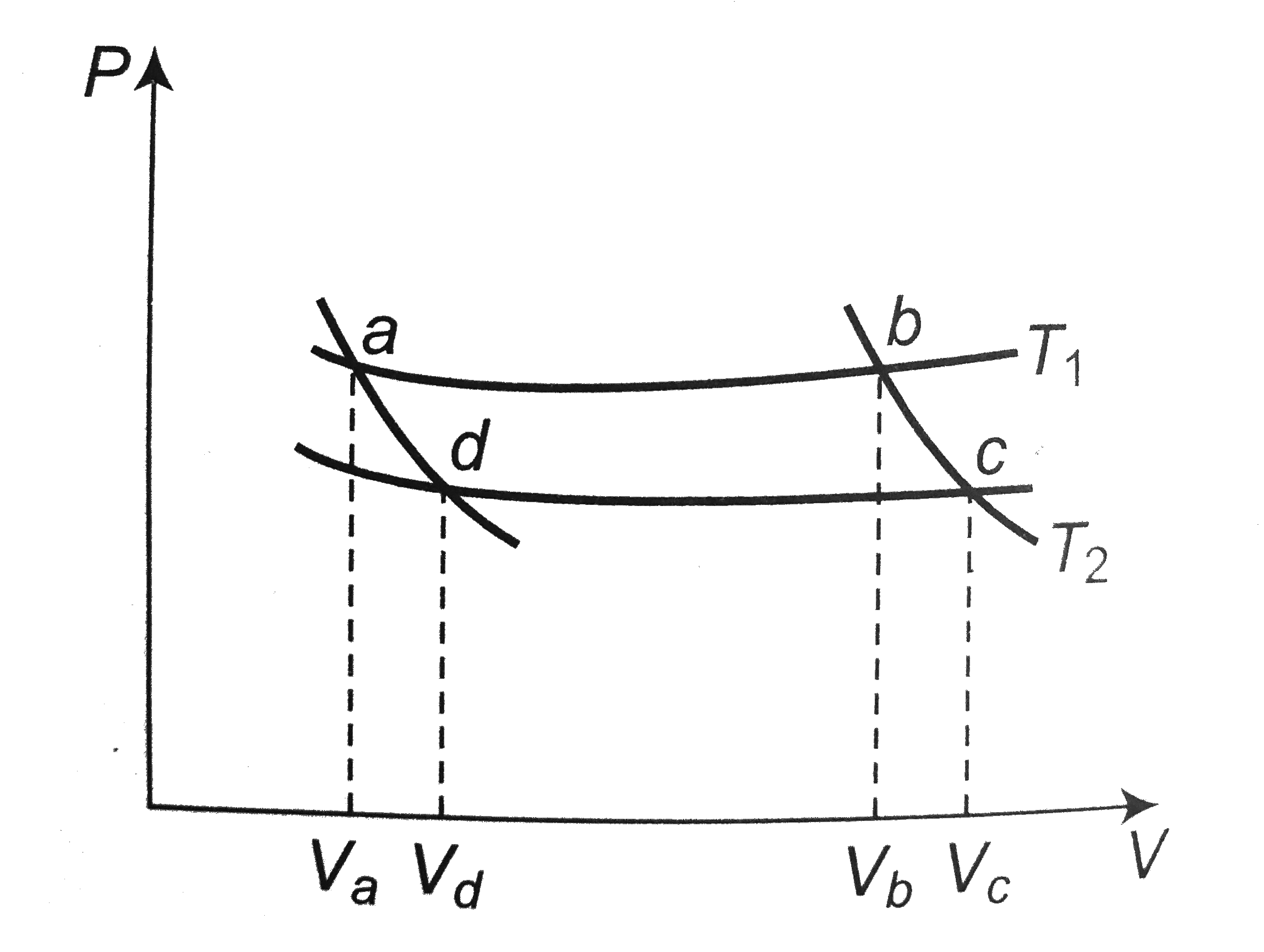
View SolutionA gas is expanded from volume V0 to 2V0 under three different processes, as shown in the figure. Process 1 is isobaric process, process 2 is isothermal and process 3 is adiabatic. Let ΔU1,ΔU2 and ΔU3 be the change in internal energy of the gas in these three processes. Then
View Solution
