The order of polarity of CH3I,CH3Br and CH3Cl molecules follows the order
A
CH3Br>CH3Cl>CH3I
B
CH3I>CH3Br>CH3Cl
C
CH3Cl>CH3Br>CH3I
D
CH3Cl>CH3I>CH3Br
Text Solution
The correct Answer is:C
Since the electronegativity follows the order Cl>Br>I, the order of polarity is CH3Cl>CH3Br>CH3I.
|
Similar Questions
Knowledge Check
The dipole moments of CH3F,CH3ClandCH3Br are in the order :
ACH3F>CH3Cl>CH3BrBCH3Cl>CH3Br>CH3FCCH3Cl>CH3F>CH3BrDCH3Br>CH3Cl>CH3FThe reactivities of CH3Cl,CH3CH2CH2ClandC6H5Cl are in the order
ACH3Cl>CH3CH2CH2Cl>C6H5ClBCH3CH2CH2Cl>CH3Cl>C6H5ClCC6H5Cl>CH3CH2CH2Cl>CH3ClDCH3Cl>C6H5Cl>CH3CH2CH2ClFor the compounds, CH3Cl,CH3I,CH3Br and
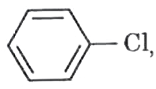 which of the following is the correct order of C-halogen bond length?A
which of the following is the correct order of C-halogen bond length?A B
B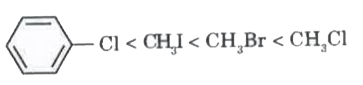 C
C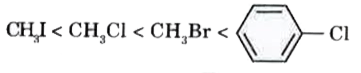 D
D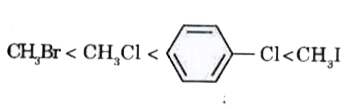
The following alkyl halidesin the decrasing order of SN2 reactivity , is
1(CH3)3CCl 2CH3Br
4 CH3Cl 4(CH3)2CHCl
View SolutionConsider the following haloakanes
1 CH3F2CH3Cl3CH2Br4CH3I
The increasing order of reactivity in nucelophilic subsittution reaction is
View SolutionThe decreasing order of dipole moment of the folliwng is:
I. CH3Cl, II. CH3Br, III. CH3F
View SolutionConsider the following haloalkanes,
I. CH3l II. CH3F
III. CH3Cl IV. CH3Br
The correct sequence of increasing order of dipole moment is
View SolutionIn SN2 reactions, the correct order of reactivity for the following compounds:
CH3CI,CH3CH2CI,(CH3)2CHCI and (CH3)2CCI is:
View Solution

