4g of mixture of Na2CO3 and NaHCO3 on heating liberates 448 ml of CO2 at STP. The percentage of Na2CO3 in the mixture is Solution in Telugu
84
16
54
80
The correct Answer is:B
Step by step video, text & image solution for 4g of mixture of Na_(2)CO_(3) and NaHCO_(3) on heating liberates 448 ml of CO_(2) at STP. The percentage of Na_(2)CO_(3) in the mixture is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- STOICHIOMETRYBook:AAKASH SERIESChapter:STOICHIOMETRYExercise:LECTURE SHEET ( EXERCISE III MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)
Explore 4 Videos
- STOICHIOMETRYBook:AAKASH SERIESChapter:STOICHIOMETRYExercise:LECTURE SHEET ( EXERCISE III LINKED COMPREHENSION TYPE QUESTIONS)
Explore 5 Videos
- STOICHIOMETRYBook:AAKASH SERIESChapter:STOICHIOMETRYExercise:LECTURE SHEET ( EXERCISE II INTEGER TYPE QUESTIONS)
Explore 8 Videos
- STATES OF MATTER Book:AAKASH SERIESChapter:STATES OF MATTER Exercise:ADDITIONAL PRACTICE EXERCISE -PRACTICE SHEET (ADVANCED) (LINKED COMPREHENSION TYPE QUESTIONS) (PASSAGE- IV)
Explore 2 Videos
- SURFACE CHEMISTRYBook:AAKASH SERIESChapter:SURFACE CHEMISTRYExercise:Objective Excersice - 4 (ASSERTION (A)& REASON (R ) TYPE QUESTION)
Explore 34 Videos
Similar Questions
Combustion of 2.24 its ethane at STP requires
View Solution10 moles of SO2 and 4 moles of O2 are mixed in a closed vessel of volume 2 litres. The mixture is heated in the presence of Pt catalyst. Following reaction takes place:
2SO2(g)+O2(g)→2SO3(g)
Assuming the reaction proceeds to completion.
Select the correct statement.
View SolutionThe oxidation number of iron in brown ring complexes +x. What is value of x.
View SolutionAt a particular temperature and pressure for a real gas Van der Waal's equation can be written as:
(P+aV2m)(Vm−b)=RT
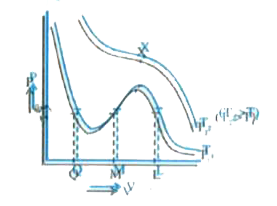
where Vm is molar volume of gas. This is cubic equation in the variable Vm and therefore for any single value of P & T there should be 3 values of Vm. Which are shown in graph as Q, M and L.
As temperature is made to increase at a certain higher temperature the three values of Vm becomes identical. The temperature, pressure & molar volume at point X are called Tc, Pc & Vc for real gas. The compressibility factor in terms of Pc, Vc and T is called Zc.
The expression of Van dcr Waal's constant 'a' can be given as
View Solution(A) De-emulsification is a reverse process of emulsion formation
(R ) During de-emulsification , the emulsion breaks to give oil and water.
View SolutionA 2.0g of mixture of Na2CO3 and NaHCO3 loses 0.248g when heated to 300∘C, the temperature at which NaHCO3 decomposes to Na2CO3, CO2, the temperature at which NaHCO3 decomposes to Na2CO3,CO3 and H2O. What is the percentage of Na2CO3 in mixture?
View Solution19g og a mixture containing NaHCO_(3) and Na_(2)CO_(3) on complete heating liberated 1,12L of CO_(2) at STP. The weight of the remaining solid was 15.9g . What is the weight (in g) of Na_(2)CO_(3) in the mixture before heating ?
View Solution25.2 gm of a mixture of NaHCO3 and Na2CO3 is heated strongly, 0.66 gm of CO2 gas is evolved then the % mass of Na2CO3 present in original mixture is -
View SolutionThe 156ml of 0.1 M HCl required to react completely with a mixture of 0.53g Na2CO3 and NaHCO3 only. What was the mass of NaHCO3 in original mixture?
Na2CO3+2HCl→2NaCl+H2CO3
NaHCO3+HCl→NaCl+H2CO3
View SolutionWhat will be the % loss in mass, if an equimolar mixture of NaHCO_(3) and Na_(2)CO_(3) is heated till constant weight?
View Solution1 g of a mixture of NaHCO_(3) and Na_(2)CO_(3) is heated to 150^(@)C . The volume of the CO_(2) produced at STP is 112.0 mL. Calculate the percentage of Na_(2)CO_(3) in the mixture (Na=23, C=12, O=16)
View Solution20 g mixture of Na_(2)CO_(3) and CaCO_(3) is gently heated. On heating this sample produces 2.24 litre of CO_(2) at STP. Calculate the % weight of Na_(2)CO_(3) in that sample.
View Solutionनिर्जल Na2CO3 तथा NaHCO3 के एक मिश्रण के द्रव्यमान में 0.372 ग्राम की कमी होती है । मिश्रण में Na2CO3 तथा NaHCO3 की प्रतिशत ज्ञात करो ।
View SolutionA 2.0g mixture of Na_(2)CO_(3) and NaHCO_(3) suffered a loss of 0.12g on heating. Percentage of Na_(2)CO_(3) in the mixture-
View SolutionThe volume of CO2 formed when a mixture of 2 mol NaHCO3and1 mol Na2CO3 at STP, is-
View Solution
