Whast is the oxidation state of Fe in the product formed when acidified potassium ferrocyanide K4[Fe(CN)6] is treated with hydrogen peroxide?Solution in Telugu
+2
+3
+1
+6
The correct Answer is:B
Step by step video, text & image solution for Whast is the oxidation state of Fe in the product formed when acidified potassium ferrocyanide K_(4)[Fe(CN)_(6)] is treated with hydrogen peroxide? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- STOICHIOMETRYBook:AAKASH SERIESChapter:STOICHIOMETRYExercise:PRACTICE SHEET (EXERCISE-II LEVEL-II STRAIGHT OBJECTIVE TYPE QUESTIONS)
Explore 10 Videos
- STOICHIOMETRYBook:AAKASH SERIESChapter:STOICHIOMETRYExercise:PRACTICE SHEET (EXERCISE-II LEVEL-II MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)
Explore 5 Videos
- STOICHIOMETRYBook:AAKASH SERIESChapter:STOICHIOMETRYExercise:PRACTICE SHEET (EXERCISE-I LEVEL-II INTEGER TYPE QUESTIONS)
Explore 10 Videos
- STATES OF MATTER Book:AAKASH SERIESChapter:STATES OF MATTER Exercise:ADDITIONAL PRACTICE EXERCISE -PRACTICE SHEET (ADVANCED) (LINKED COMPREHENSION TYPE QUESTIONS) (PASSAGE- IV)
Explore 2 Videos
- SURFACE CHEMISTRYBook:AAKASH SERIESChapter:SURFACE CHEMISTRYExercise:Objective Excersice - 4 (ASSERTION (A)& REASON (R ) TYPE QUESTION)
Explore 34 Videos
Similar Questions
An element A in a compound AB has oxidation number -n. IT is oxidized by Cr2O2−7 in acid medium. In the experiment 1.68×10−3 moles of K2Cr2O7 was used for 3.36×10−3 moles of AB. The new oxidation number of A after oxidation is
View SolutionThe oxidation number of carbon is zero is
View SolutionRahul Dravid wants to wear 6.023×1021 Ag atms in the form of a ring. His Silver Gold Copper alloy ring consists of 20% of Silver. The mass of the ring is 0.9 x. What is x?
View SolutionAt a particular temperature and pressure for a real gas Van der Waal's equation can be written as:
(P+aV2m)(Vm−b)=RT
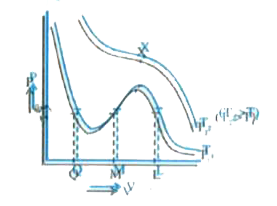
where Vm is molar volume of gas. This is cubic equation in the variable Vm and therefore for any single value of P & T there should be 3 values of Vm. Which are shown in graph as Q, M and L.
As temperature is made to increase at a certain higher temperature the three values of Vm becomes identical. The temperature, pressure & molar volume at point X are called Tc, Pc & Vc for real gas. The compressibility factor in terms of Pc, Vc and T is called Zc.
The expression of Van dcr Waal's constant 'a' can be given as
View Solution(A) De-emulsification is a reverse process of emulsion formation
(R ) During de-emulsification , the emulsion breaks to give oil and water.
View SolutionWhat is the oxidation state of Fe in the product formed when acidified Potasssium ferrocyanide (K_(4)[Fe(CN)_(6)]) is treated with hydrogen peroxide?
View SolutionIn K_(4)[Fe(CN)_(6)],Fe is in the form of
View SolutionHydrogen peroxide oxidises [Fe(CN)6]4− to [Fe(CN)6]3− in acidic medium but reduces [Fe(CN)6]3− to [Fe(CN)6]4− in alkaline medium. The other products formed are, respectively
View SolutionThe oxidation state of Fe in K_(4)[Fe(CN)_(6)] is
View Solutionहाइड्रोजन परॉक्साइड अम्लीय माध्यम में, [Fe(CN)6]4− को [Fe(CN)6]3− में ऑक्सीकृत करता है परन्तु क्षारीय माध्यम में [Fe(CN)6]3− को [Fe(CN)6]4− में अपचयित करता है। अन्य बनने वाले उत्पाद क्रमशः है -
View SolutionWhat is the oxidation state of Fe in the product formed when acidified potassium ferrocyanide is treated with H_(2)O_(2) ?
View SolutionHydrogen peroxide oxidises [Fe(CN)6]4−→[Fe(CN)6]3− in acidic medium but reduces [Fe(CN)6]3− to [Fe(CN)6]4− in alkaline medium. The other products formed are, respectively-
View SolutionHydrogen peroxide oxidises [Fe(CN)_(6)]^(4-) to [Fe (CN)_(6)]^(3-) in acidic medium but reduces [Fe(CN)_(6)]^(3-) to [Fe(CN)_(6)]^(4-) in alkaline medium . The other products formed are , respectively -
View SolutionThe oxidation state of Fe in [Fe(CN)_(6)]^(4+) is…………………..
View SolutionOxidation state of Fe in K4[Fe(CN)6]
View Solution
