Study the following isotherms of carbondioxide at various temperature and answer the following questions based on that:
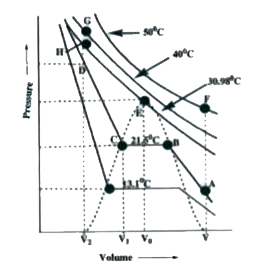
Choose the temperature above which CO2 cannot be liquified whatsoever applied pressure isSolution in Telugu
290K
294.5 K
302K
304 K
Step by step video & image solution for Study the following isotherms of carbondioxide at various temperature and answer the following questions based on that: Choose the temperature above which CO_(2) cannot be liquified whatsoever applied pressure is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- STATES OF MATTER Book:AAKASH SERIESChapter:STATES OF MATTER Exercise:ADDITIONAL PRACTICE EXERCISE (LEVEL-II LECTURE SHEET (ADVANCED)) (LINKED COMPREHENSION TYPE QUESTIONS) (PASSAGE-II)
Explore 3 Videos
- STATES OF MATTER Book:AAKASH SERIESChapter:STATES OF MATTER Exercise:ADDITIONAL PRACTICE EXERCISE (LEVEL-II LECTURE SHEET (ADVANCED)) (MATRIX MATCHING TYPE QUESTIONS)
Explore 1 Video
- STATES OF MATTER Book:AAKASH SERIESChapter:STATES OF MATTER Exercise:ADDITIONAL PRACTICE EXERCISE (LEVEL-II LECTURE SHEET (ADVANCED)) (MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)
Explore 5 Videos
- SOLUTIONS Book:AAKASH SERIESChapter:SOLUTIONS Exercise:Objective Exercise -4
Explore 50 Videos
- STOICHIOMETRYBook:AAKASH SERIESChapter:STOICHIOMETRYExercise:PRACTICE SHEET (ADVANCED) (INTEGER TYPE QUESTION)
Explore 2 Videos
Similar Questions
In a various form of matter the constituent particles are held together with different types of forces. The extent of forces depends on the nature of the substances.
The forces operating in solid xenon are________
View Solution
View SolutionP and Q are ideal gases which do not react with each other. The mass of one mole of P is four times that of Q. At STP, which of the following are true?
View SolutionAssertion : Molarity of a solution decreases with an increase of temperature
Reason : As temperature increases volume of the solution increases.
View SolutionAmmonia is oxised by oxygen to give nitric oxide and water. The weight of water produced per gram of nitric oxide is 0.1×xg. What is value of d.
View SolutionIsotherms of carbon dioxide at various temperatures are represented in figure. Answer the following questions based on this figure. In which state will CO_(2) exist between the points a and b at temperature T_(1) ?
View SolutionIsotherms of carbon dioxide at various temperatures are represented in figure. Answer the following questions based on this figure. At what point will CO_(2) start liquefying when temperature is T_(1) ?
View SolutionIsotherms of carbon dioxide at various temperatures are represented in figure. Answer the following questions based on this figure.
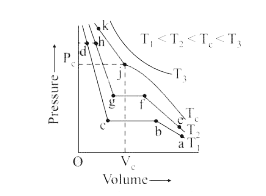
At what point will CO2 be completely liquefied when temperature is T2 ?
View SolutionIsotherms of carbon dioxide at various temperatures are represented in figure. Answer the following questions based on this figure.
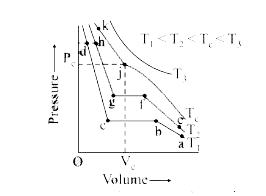
Will condensation take place when the temperature is T3?
View SolutionIsotherms of carbon dioxide at various temperatures are represented in figure. Answer the following questions based on this figure. What portion of the isotherm at T_(1) represent liquid and gaseous CO_(2) at equilibrium?
View SolutionAnswer the following questions based on the P-T phase diagram of CO2:
CO2 at 1 am pressure and temperature −60∘C is compressed isothermally Does it go through a liquid phase ?
View SolutionAnswer the following questions based on the P-T phase diagram of CO_(2) : CO_(2) at 1 am pressure and temperature - 60^(@) C is compressed isothermally Does it go through a liquid phase ?
View SolutionStudy the following isotherms of carbondioxide at various temperature and answer the following questions based on that:
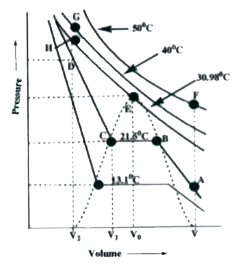
At 21.5°C decrease in volume(B-C) doesn't result into increase in pressure because
View SolutionStudy the following isotherms of carbondioxide at various temperature and answer the following questions based on that:
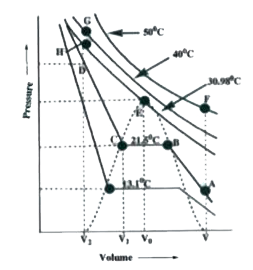
Carbondioxide present at F can be liquified
View SolutionStudy the following isotherms of carbondioxide at various temperature and answer the following questions based on that:

Choose the temperature above which CO2 cannot be liquified whatsoever applied pressure is
View Solution
