An electrochemical cell consists of two metallic electrodes dipping in _________ solution(s).
electrolytic
Topper's Solved these Questions
- ELECTROCHEMISTRY BOOK - U-LIKE SERIESCHAPTER - ELECTROCHEMISTRY EXERCISE - CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (TRUE OR FALSE )
8 Videos
- ELECTROCHEMISTRY BOOK - U-LIKE SERIESCHAPTER - ELECTROCHEMISTRY EXERCISE - CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (VERY SHORT ANSWER QUESTIONS (1 MARK EACH))
47 Videos
- ELECTROCHEMISTRY BOOK - U-LIKE SERIESCHAPTER - ELECTROCHEMISTRY EXERCISE - CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (ASSERTION-REASON QUESTIONS )
10 Videos
- COORDINATION COMPOUNDSBOOK - U-LIKE SERIESCHAPTER - COORDINATION COMPOUNDSEXERCISE - SELF ASSESSMENT TEST
7 Videos
- EXAMINATION PAPER 2020BOOK - U-LIKE SERIESCHAPTER - EXAMINATION PAPER 2020EXERCISE - SECTION D
15 Videos
Similar Questions
NaCl, CaCl2,MgSO4 are known as 1 - 1, 2 - 1 and 2 - 2 elecrolytes respectively.
View SolutionWrite the reduction and oxidation half reactions for Daniell cell.
View SolutionAssertion (A) : Electrochemistry is the study of production of electricity from energy released during spontaneous chemical reactions.
Reason (R) : Study of electrochemistry is important for creating new technologies.
View SolutionWhich type of shapes are uncommon in coordination compounds ?
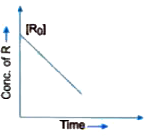
View SolutionVisha plotted a graph between concentration of R and time for a reaction R→P. On the basis of this graph, answer the following question :
(i) Predict the order of reaction.
(ii) What does the slope of the line indicate ?
(iii) What are the units of rate constant ?
[Give :log2=0.3010,log3=0.4771,log4=0.6021]
View SolutionA silver electrode dipping in AgNO3 solution (0.1M) is combined salt bridge with a hydrogen electrode dipping in a solution of pH=3(at25∘C). If the standard reduction potential of the silver electrode is 0.799V, what is the EMF of the cell ?
View SolutionIf hydrogen electrodes dipped in two solutions of pH=3 and pH=6 are connected by a salt bridge, the EMF_(cell) is
View SolutionThe EMF of concentration cell consisting of two zinc electrodes, one dipping into M/4 solution of ZnSO4 and the other into M/16 solution of the same salt at 25∘C is
View SolutionIn electrochemical cells, cathode is always the electrode where:
View SolutionIn an electrochemical cell consisting of zinc eelctrode and normal hydrogen electrode zinc electrode acts as ……………………
View SolutionIn an electrochemical cell consisting of zinc eelctrode and normal hydrogen electrode zinc electrode acts as ……………………
View SolutionThe EMF of a galvanic cell consisting of two hydrogen electrodes is 0.17 V. If the solution of one of the electrodes has [H+]=10−3 M, the pH at the other electode is
View Solutionelectrochemical cells: various types of electrodes and their potentials
View SolutionAn electrochemical cell consists of two electrodes i.e., Anode and Cathode. What is the direction of flow of electrons in this cell ?
View SolutionA cell consists of two hydrogen electrode . The negative electrode is in contact with a solution having pH = 6 . The positive electrode is in contact with a solution of pH = x . Calculate the value of x if the e.m.f. of the cell is found to be 0.118 V at 298 K .
View Solution

