Consider the figure below and answer the following questions :
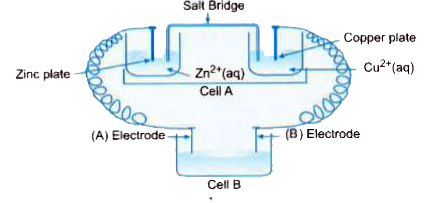
(i) Cell 'A' has Ecell=2V and Cell 'B' has Ecell=1.1V which of the two cells 'A' or 'B' will act as an electrolytic cell. Which electrode reaction will occur in this cell?
(ii) If cell 'A' has Ecell=0.5V and cell 'B' has Ecell=1.1V then what will be the reaction at anode and cathode?
(i) Cell .B. will act as electrolytic cell as it has lower emf.
The electrode reactions will be :
Zn2++2e−→Zn at cathode
Cu→Cu2++2e− at anode.
(ii) Now cell .B. acts as galvanic cell as it has higher emf and will push electrons into cell .A.. The electrode reaction will be :
At anodeZn→Zn2++2e−At cathodeCu2++2e−→Cu
Topper's Solved these Questions
- ELECTROCHEMISTRY Book:U-LIKE SERIESChapter:ELECTROCHEMISTRY Exercise:CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (LONG ANSWER QUESTIONS-II (5 MARKS EACH))
Explore 16 Videos
- ELECTROCHEMISTRY Book:U-LIKE SERIESChapter:ELECTROCHEMISTRY Exercise:SELF ASSESSMENT TEST (SECTION A) MULTIPLE CHOICE QUESTIONS (CHOOSE THE CORRECT OPTION)
Explore 7 Videos
- ELECTROCHEMISTRY Book:U-LIKE SERIESChapter:ELECTROCHEMISTRY Exercise:CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (SHORT ANSWER QUESTIONS (2 MARKS EACH))
Explore 47 Videos
- COORDINATION COMPOUNDSBook:U-LIKE SERIESChapter:COORDINATION COMPOUNDSExercise:SELF ASSESSMENT TEST
Explore 7 Videos
- EXAMINATION PAPER 2020Book:U-LIKE SERIESChapter:EXAMINATION PAPER 2020Exercise:SECTION D
Explore 15 Videos
Similar Questions
Consider the figure below and answer the following questions :
(i) Cell 'A' has Ecell=2 V and Cell B has Ecell=1.1 V which of the two cells 'A' or 'B' will act as an electrolytic cell. Which electrode reactions will occur in this cell ?
(ii) If cell 'A' has Ecell=0.5 V and cell 'B' has Ecell=1.1 V then what will be the reactions at anode and cathode ?

View SolutionConsider the figure and answer the following question.
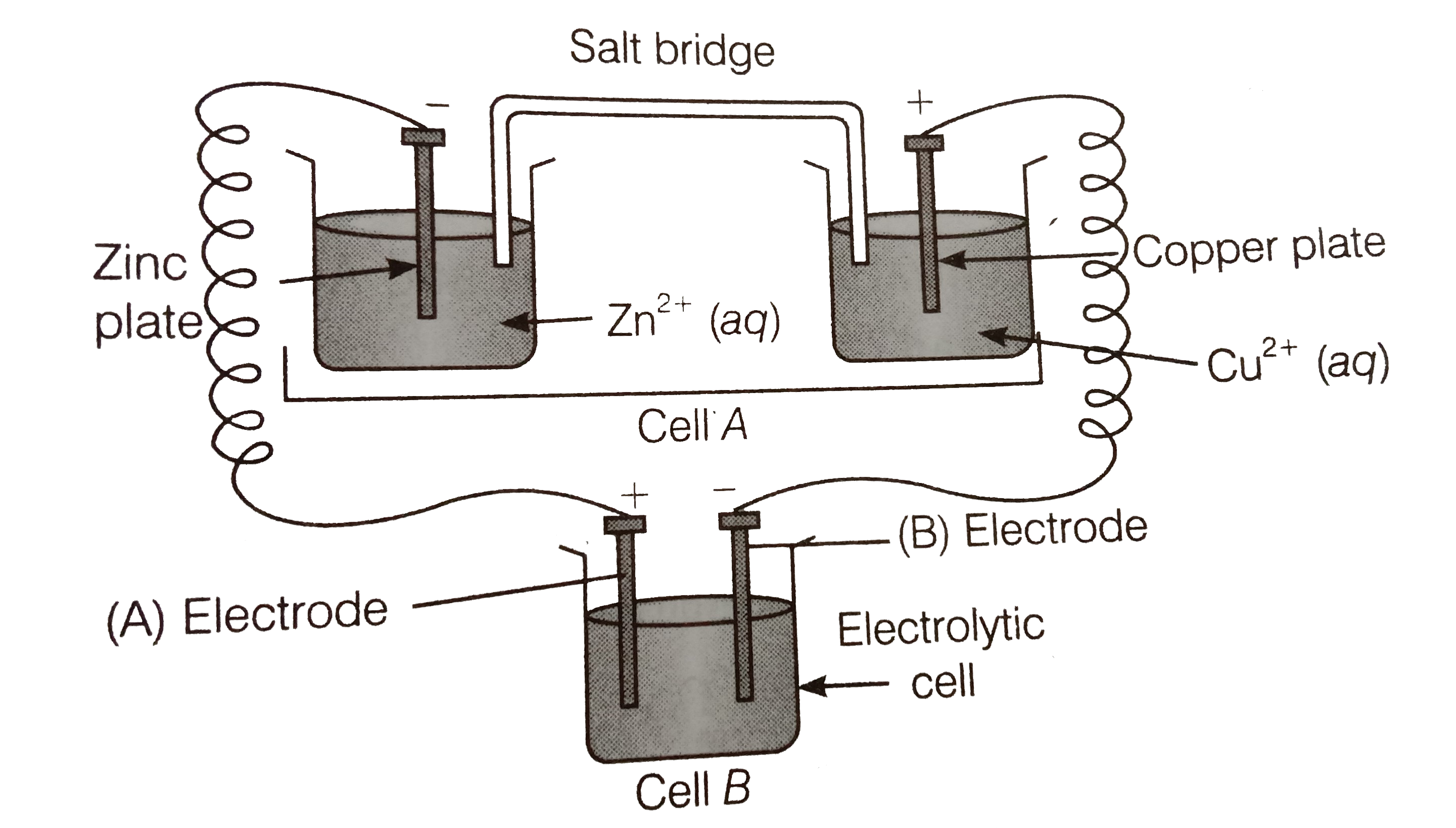
(i) Cell 'A' h as Ecell=2V and Cell 'B' has Ecell=1.1V which of the two cell 'A' or 'B' will act as an electrolytic cell. Which electrode reactions will occur in this cell?
(ii) If cell 'A' has Ecell= 0.5V and cell 'B' has Ecell=1.1V then what will be the reactions at anode and cathode?
View Solution
Knowledge Check
In which of the following cell(s): Ecell=E∘cell?
ACu(s)∣∣Cu2+(0.01M)∣∣∣∣Ag+(0.1M)∣∣Ag(s)BPt(H2)|pH=1|∣∣Zn2+(0.01M)∣∣∣Zn(s)CPt(H2)|pH=1|∣∣Zn2+(1M)∣∣∣Zn(s)DPt(H2)∣∣H+=0.1M∣∣∣∣Zn2+(0.01)∣∣∣∣Zn(s)
Consider the figure and answer the following question.

(i) Cell 'A' h as Ecell=2V and Cell 'B' has Ecell=1.1V which of the two cell 'A' or 'B' will act as an electrolytic cell. Which electrode reactions will occur in this cell?
(ii) If cell 'A' has Ecell= 0.5V and cell 'B' has Ecell=1.1V then what will be the reactions at anode and cathode?
View SolutionIn which of the following cell(s): Ecell=E∘cell?
View SolutionConsider the following diagram in which an electrochemical cell is coupled to an electrolytic cell. What will be the polarity of electrodes 'A' and 'B' in the electrolytic cell ?

View SolutionConsider the following diagram in which an electrochemical cell is coupled to an electrolytic cell. What will be the polarity of electrodes 'A' and 'B' in the electrolytic cell ?
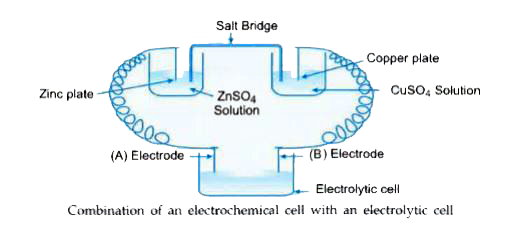
View SolutionConsider the following diagram in which an electrochemical cell is coupled to an electrolytic cell. What will be the polarity of electrodes 'A' an d 'B' in the electrolytic cell?
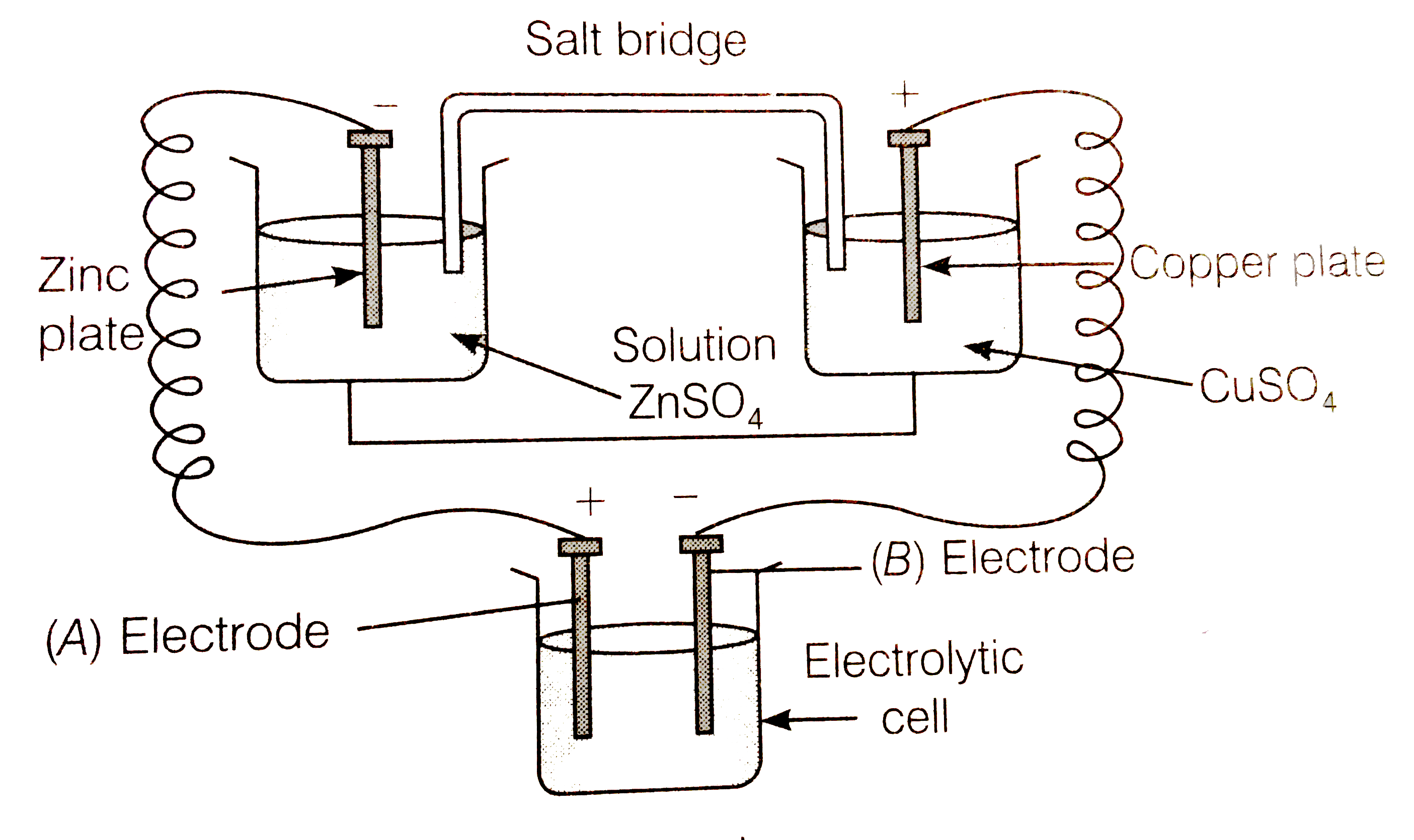
View SolutionConsider the following diagram in which an electrochemical cell is coupled to an electrolytic cell. What will be the polarity of electrodes 'A' an d 'B' in the electrolytic cell?

View SolutionE∘cell=1.1V for Daniel cell. Which of the following expressions are correct description of state of equilibrium in this cell?
View Solution

