How would you account for the following:
NCI3 is an endothermic compound while NF3 is an exothermic one.
Heat is absorbed when NCl3 is formed from N2andCl2. Heat is evolved when NF3 is formed from N2andF2.
Topper's Solved these Questions
- THE P-BLOCK ELEMENTS Book:U-LIKE SERIESChapter:THE P-BLOCK ELEMENTS Exercise:LONG ANSWER QUESTION-II
Explore 68 Videos
- THE P-BLOCK ELEMENTS Book:U-LIKE SERIESChapter:THE P-BLOCK ELEMENTS Exercise:SELF ASSESSMENT TEST ( SECTION A)
Explore 7 Videos
- THE P-BLOCK ELEMENTS Book:U-LIKE SERIESChapter:THE P-BLOCK ELEMENTS Exercise:SHORT ANSWER QUESTIONS
Explore 87 Videos
- THE D-AND F-BLOCK ELEMENTS Book:U-LIKE SERIESChapter:THE D-AND F-BLOCK ELEMENTS Exercise:SELF ASSESSMENT TEST (SECTION A ) MULTIPLE CHOICE QUESTIONS (CHOOSE THE CORRECT OPTION)
Explore 7 Videos
Similar Questions
NCl3 is an endothermic compound
while NF3 is an exothermic compound.
View SolutionNCl3 is an endothermic compound while NF3 is an exothermic compound.
View SolutionHow would you account for the following ?
NF3 is an exothermic compound but NCI, is endothermic compound.
View SolutionExplain the following:
NF3 is an exothermic compound whereas NCl3 is not.
View SolutionHow would you name the following compound?
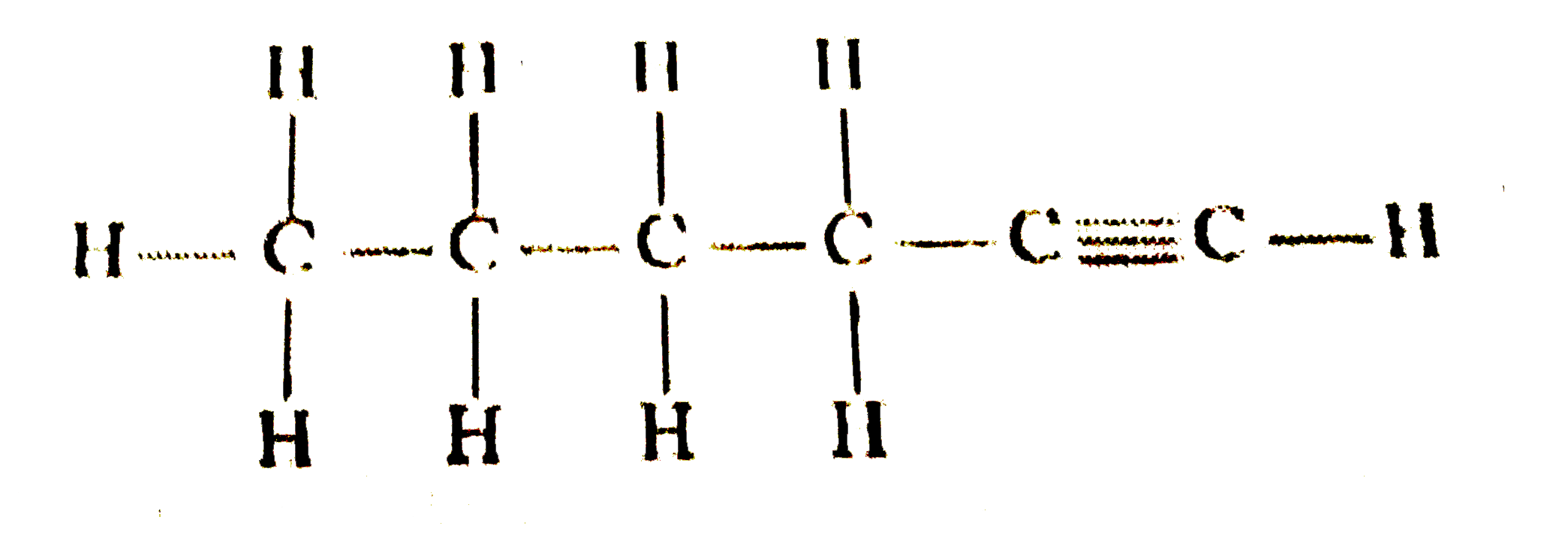
View SolutionExplain the following :
(a) The electron gain enthalpy with negative sign for fluorine is less than for chlorine, still fluorine is a stronger oxidising agent than chlorine.
(b) XeF2 is linear molecule without a bend.
(c) NCl3 is an endothermic compound while NF3 is an exothermic one.
View SolutionExplain the following :
(a) The electron gain enthalpy with negative sign for fluorine is less than for chlorine, still fluorine is a stronger oxidising agent than chlorine.
(b) XeF2 is linear molecule without a bend.
(c) NCl3 is an endothermic compound while NF3 is an exothermic one.
View SolutionHow would you account for the following :
Ni(CO)4 possesses tetrahedral geometry while [Pt(NH3)2Cl2] is square planar.
View SolutionHow would you account for the following ?
ClF3 molecule has a T-shaped structure and not a trigonal planar one.
View SolutionHow would you account for the following :
[Fe(CN)6]3− is weakly paramagnetic while [Fe(CN)6]4− is diamagnetic
View Solution

