(a) Complete the following chemical equations :
(i) Cr2O2−7(aq)+H2S(g)+H+(aq)→
(ii) Cu2+(aq)+I−(aq)→
(b) How would you account for the following:
(i) The oxidising power of oxoanions are in the order
VO+2<Cr2O2−7<MnO−4.
(ii) The third ionisation enthalpy of manganese (Z = 25) is exceptionally high.
(iii) Cr2+ is a stronger reducing agent than Fe2+.
(a) (i) 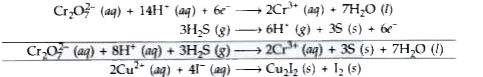
(ii) 2Cu2+(aq)+4I−(aq)→Cu2I2(s)+I2(s)
(b) (i) Oxidising power of oxoanions is in the order :
VO+2<Cr2O2−7<MnO−4
This is due to increasing stability of the lower species to which they are reduced.
(ii) This is due to exceptional stability of Mn2+(3d5) which is due to highly stable half-filled configuration.
(iii) Cr2+ is a stronger reducing agent than Fe2+. This is because after losing one electron Cr2+(d4) changes to Cr3+(d3) and these electrons are accommodated in lower t2g orbitals which gives Cr3+ greater stability. On the other hand, Fe2+(d6) on losing one electron change to Fe3+(d5). Although half-filled, the configuration is comparative less stable than Cr3+.
Topper's Solved these Questions
- THE D-AND F-BLOCK ELEMENTS Book:U-LIKE SERIESChapter:THE D-AND F-BLOCK ELEMENTS Exercise:SELF ASSESSMENT TEST (SECTION A ) MULTIPLE CHOICE QUESTIONS (CHOOSE THE CORRECT OPTION)
Explore 7 Videos
- THE D-AND F-BLOCK ELEMENTS Book:U-LIKE SERIESChapter:THE D-AND F-BLOCK ELEMENTS Exercise:LONG ANSWER QUESTIONS-I (3 marks each)
Explore 22 Videos
- SURFACE CHEMISTRYBook:U-LIKE SERIESChapter:SURFACE CHEMISTRYExercise:SELF ASSESSMENT TEST ( SECTION A)
Explore 7 Videos
- THE P-BLOCK ELEMENTS Book:U-LIKE SERIESChapter:THE P-BLOCK ELEMENTS Exercise:SELF ASSESSMENT TEST ( SECTION A)
Explore 7 Videos
Similar Questions
(a) Complete the following chemical equations:
(i) Cr2O2−7(aq)+H2S(g)+H+(aq)→
(ii) Cu2+(aq)+I−(aq)→
(b) How would you account for the following :
(i) The oxidising power of oxoanions are in the order VO+2<Cr2O2−7<MnO−4
(ii) The third ionization enthalpy of manganess (Z = 25) is exceptionally high.
(iii) Cr2+ is a stronger reducing agent than Fe2+.
View Solution(a) Complete the following chemical equation:
(i) Cr2O2−7(aq)+H2S(g)+H+(aq)→ ,
(ii) Cu2+(aq)+I−(aq)→
(b) How would you account for the following:
(i)The oxidising power of oxoanion are in the orderVO+2<Cr2O2−7<MnO−4.
(ii) The third ionization enthalpy of manganese (Z=25) is exceptionally high.
(iii) Cr2+ is a stronger reducing agent than Fe2+.
Or
(a) Complete the following chemical equation:
(i) MnO−4(aq)+s2O2−3(aq)+H2O(I)→
(ii) Cr2O2−7(aq)+Fe2+(aq)+H+(aq)→
(b) Explain the following observation:
(i) La3+(Z=57) and Lu3+(Z=71) do not show any colour in solutions.
(ii) Among the divalent cation in the first series of transition elements, manganese exhibits the maximum paramagnetism.
(iii) Cu+ ion is not known in aqueous solutions.
View Solution(a) Complete the following chemical equations:
(i) Cr2O2−7(aq)+H2S(g)+H+(aq)→
(ii) Cu2+(aq)+I−(aq)→
(b) How would you account for the following :
(i) The oxidising power of oxoanions are in the order VO+2<Cr2O2−7<MnO−4
(ii) The third ionization enthalpy of manganess (Z = 25) is exceptionally high.
(iii) Cr2+ is a stronger reducing agent than Fe2+.
View SolutionComplete the following chemical equations :
(i) Cr2O2−7+H++I−→
(ii) MnO−4+NO−2+H+→
View SolutionComplete the following chemical equations :
(i) Cr2O−7+H++I−→
(ii) MnO−4+NO−2+H+→
View SolutionComplete the chemical equations :
MnO−4(aq)+S2O2−3(aq)+H2O(l)→
View SolutionComplete the chemical equations :
Cr2O2−7(aq)+Fe2+(aq)+H+(aq)→
View SolutionComplete the following chemical equation:
(i) MnO−4(aq)+s2O2−3(aq)+H2O(I)to (ii) MnO−4+NO−2+H+to
View SolutionComplete the following chemical equations :
(i)P4(s)+NaOH(aq)+H2O(l)→
I−(aq)+H2O(l)+O3(g)→
View SolutionComplete the following chemical equations :
(i) MnO−4(aq)+S2O2−3(aq)+H2O(l)→
(ii) Cr2O2−7(aq)+Fe2+(aq)+H+(aq)→
View Solution

