Account for the following :
Aniline is a weaker base compared to ethanamine.
Aniline is a weaker base compared to ethanamine :
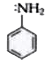
The lone pair of electrons takes part in resonance with the benzene ring. The electrons are not available on nitrogen. Therefore aniline is a weaker base.
Topper's Solved these Questions
Similar Questions
Account for the following :
(i) Aniline is weaker base than methylamine.
(ii) Aryl cyanides cannot be formed by the reaction of aryl halides and sodium cyanide.
View SolutionExplain the following:
Aniline is a weaker base than ethylamine.
View SolutionAccount for the following :
pKb of aniline is more than that of methylamine.
View SolutionAccount for the following:
Aniline is acylated first to prepare its monobromo derivative.
View SolutionAniline is a weaker base than benzyl amine.
View SolutionAniline is a weaker base than cyclohexylamine. Explain.
View SolutionAniline is a weaker base than cyclohexylamine. Explain.
View SolutionAccount for the following :
Aniline does not undergo Friedel-Crafts reaction.
View SolutionAccount for the following :
Aniline does not give friedel crafts reaction
View SolutionGive reason for the following:
PH3 is a weaker base than NH3
View Solution

