A first order reaction takes 25 minutes for 25% decomposition. Calculate t1/2.
[Give :log2=0.3010,log3=0.4771,log4=0.6021]
Apply the relation :
k=2.303tlog[R]0[R]
25% decomposition means [R]=0.75[R]0 and t=25 minutes Substituting the values in the equation above,, we have
k=2.30325log[R]00.75[R]0
=2.30325log43
=2.30325[log4−log3]
=2.30325[0.6021−0.4771]
=2.30325×0.1250=0.011515 minutes−1
t1/2=0.693k
=0.6930.011515 minutes−1
=60.18 minutus
Topper's Solved these Questions
Similar Questions
Knowledge Check
A first order reaction takes 40 min for 30% decomposition. Calculate t1/2. (Given log7=0.845)
A33.2minB77.7minC22.7minD40min
A first order decomposition reaction takes 40 minutes for 30% decomposition. Calculate its t1/2 value.
View SolutionA first order decomposition reaction takes 40 minutes for 30% decomposition. Calculate its t1/2 value.
View SolutionA first order reaction takes 20 minutes for 25% decomposition. Calculate the time when 75% of the reaction will be completed.
(Given, log2=0.3010,log3=0.4771,log4=0.6021)
View SolutionA first order reaction takes 10 minutes for 25% decomposition. Calculate half life period of the reaction.
View SolutionA first order reaction takes 20 minutes for 25% decomposition. Calculate the time when 75% of the reaction will be completed.
(Given, log2=0.3010,log3=0.4771,log4=0.6021)
View SolutionA first order reaction takes 20 minutes for 25% decomposition. Calculate the time when 75% of the reaction will be completed. [Given : log 2 = 0.3010, log 3 = 0.4771, log 4 = 0.6021]
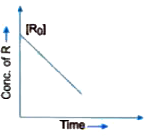
View SolutionVisha plotted a graph between concentration of R and time for a reaction R→P. On the basis of this graph, answer the following question :
(i) Predict the order of reaction.
(ii) What does the slope of the line indicate ?
(iii) What are the units of rate constant ?
[Give :log2=0.3010,log3=0.4771,log4=0.6021]
View Solution

