For a reaction A+2B→2C, the kinetic data is given below:
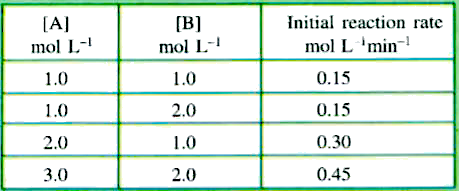
Determine the order of the reaction and write the rate law.Solution in Telugu
The correct Answer is:rate = K[A]
Step by step video, text & image solution for For a reaction A + 2B to 2C, the kinetic data is given below: Determine the order of the reaction and write the rate law. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
For the reaction : A+2B→P the rate law is given by rate =k[A][B]2
From the following data , calculate x and y .
 .
.
View SolutionThe rate law of the reaction, 2A+B→2AC is represented as Rate =K[A]2[B]. If A is taken in large excess, the order of the reaction will be,
View SolutionThe rate law for the single step reaction 2A + B to 2C is given by
View SolutionWrite the rate law for a first order reaction.
View SolutionForm the data given below for the reaction: A to B to Products the rate law expression is:
View SolutionChemcial Kinetics | Rate Law and Order Of Reaction
View SolutionThe data given below is for the reaction,
2N2Og(g)→4NO2(g)+O2(g)
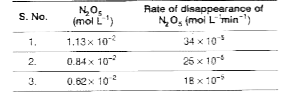
Determine for the above reaction,
rate law
View SolutionThe rate law for the single step reaction 2A + B to 2C is given by
View Solution

