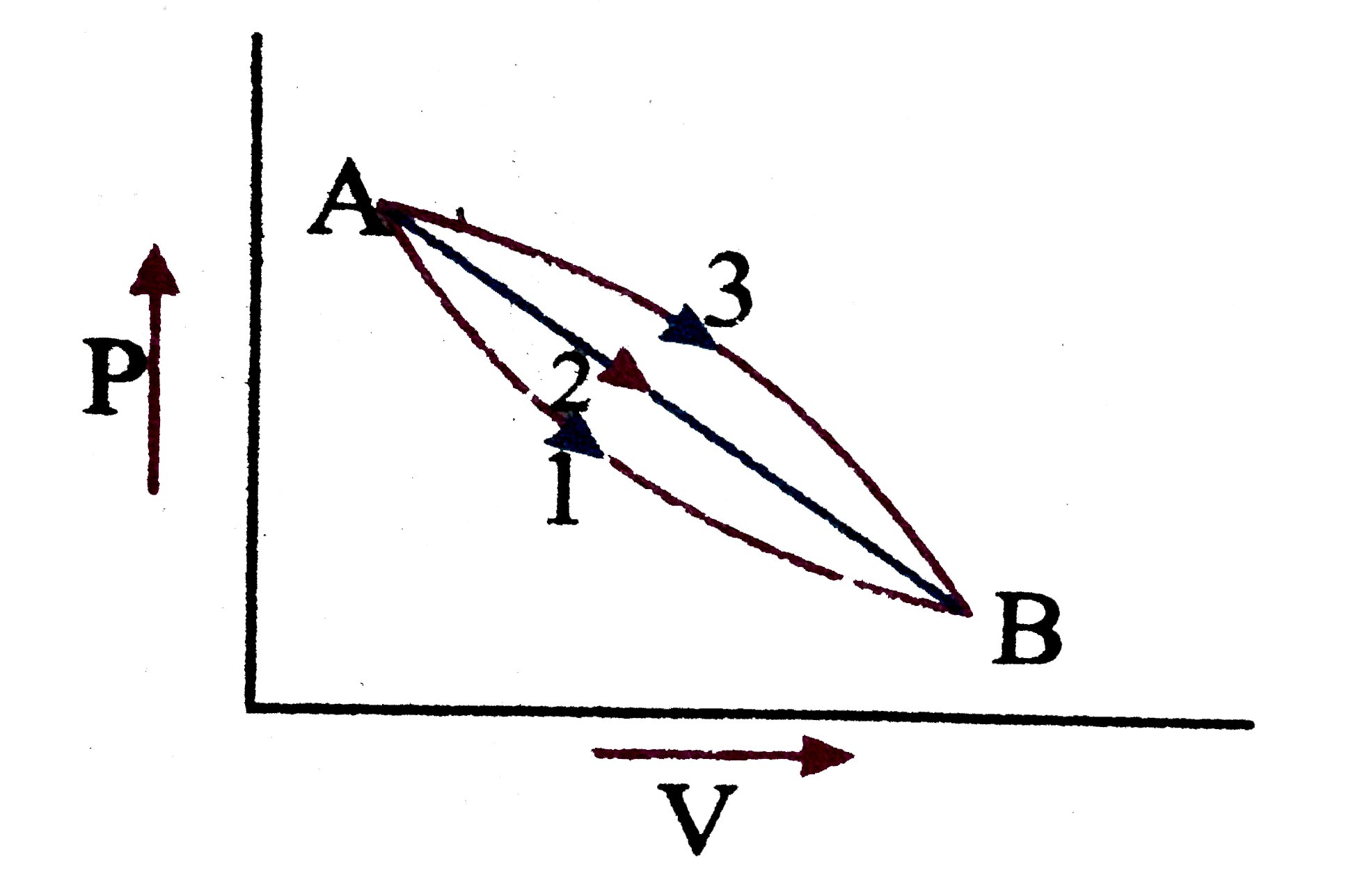
A given mass of gas expands from the state A to the state B by three paths 1,2 and 3 as shown in the figure. If w1,w2andw3 respectively be the work done by the gas along three paths then:
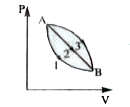 Solution in Telugu
Solution in Telugu
w1>w2>w3
w1<w2<w3
w1=w2=w3
w2<w3<w1
Step by step video & image solution for A given mass of gas expands from the state A to the state B by three paths 1,2 and 3 as shown in the figure. If w_(1), w_(2) and w_(3) respectively be the work done by the gas along three paths then: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
A given mass of gas expands from state A to state B by three paths 1, 2 and 3 as shown in the figure
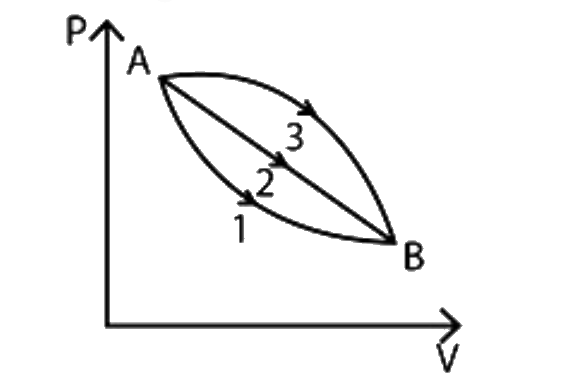
If w1,w2andw3 respectively are be the works done by the gas along three paths, then
View SolutionA given mass of gas expands from state A to state B by three paths 1,2, and 3 as shown in the figure below. If w1,w2 and w3, respectively, be the work done by the gas along three paths, then
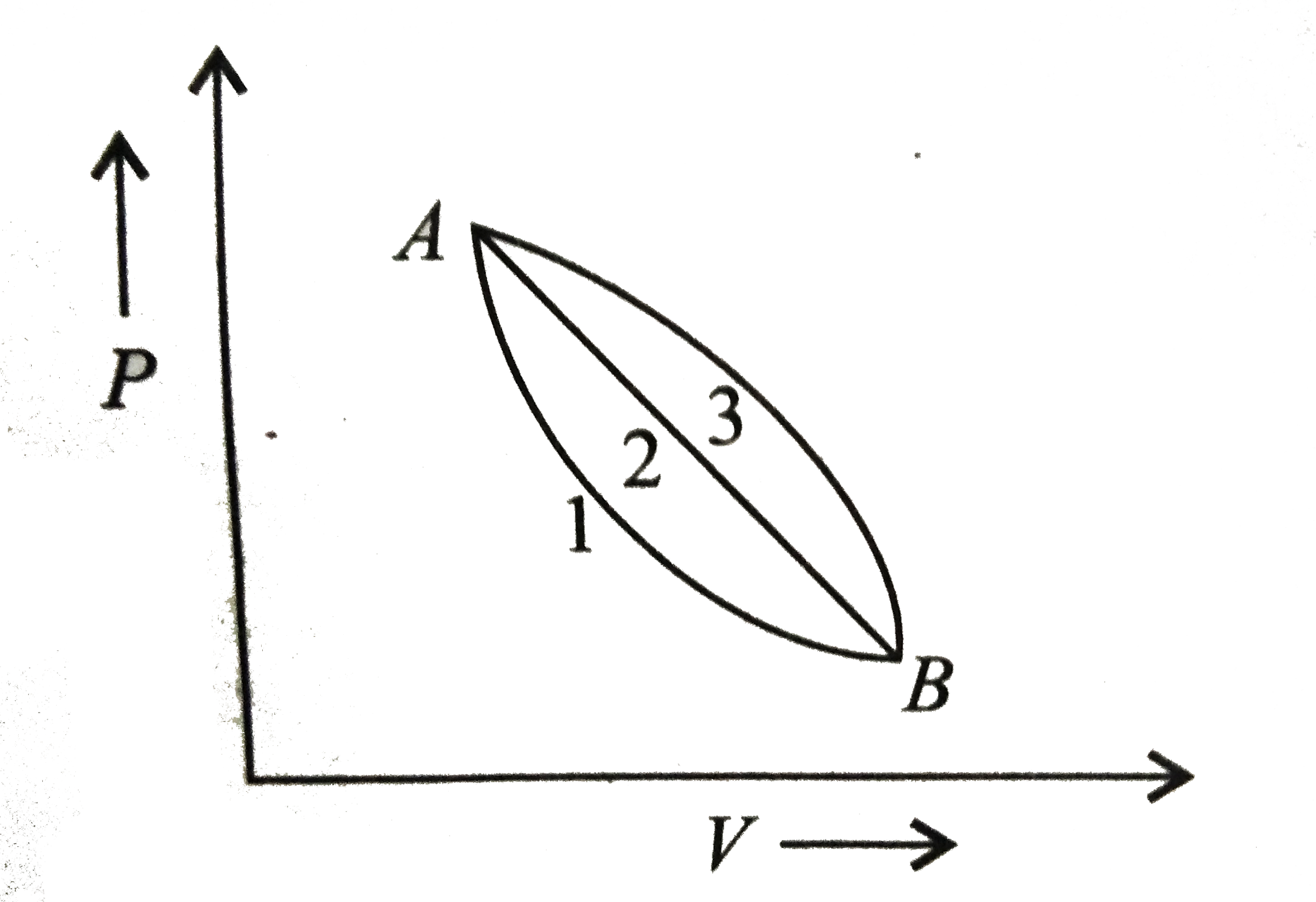
View SolutionA given mass of gas expands from state A to state B by three paths 1, 2, and 3 as shown in the figure :
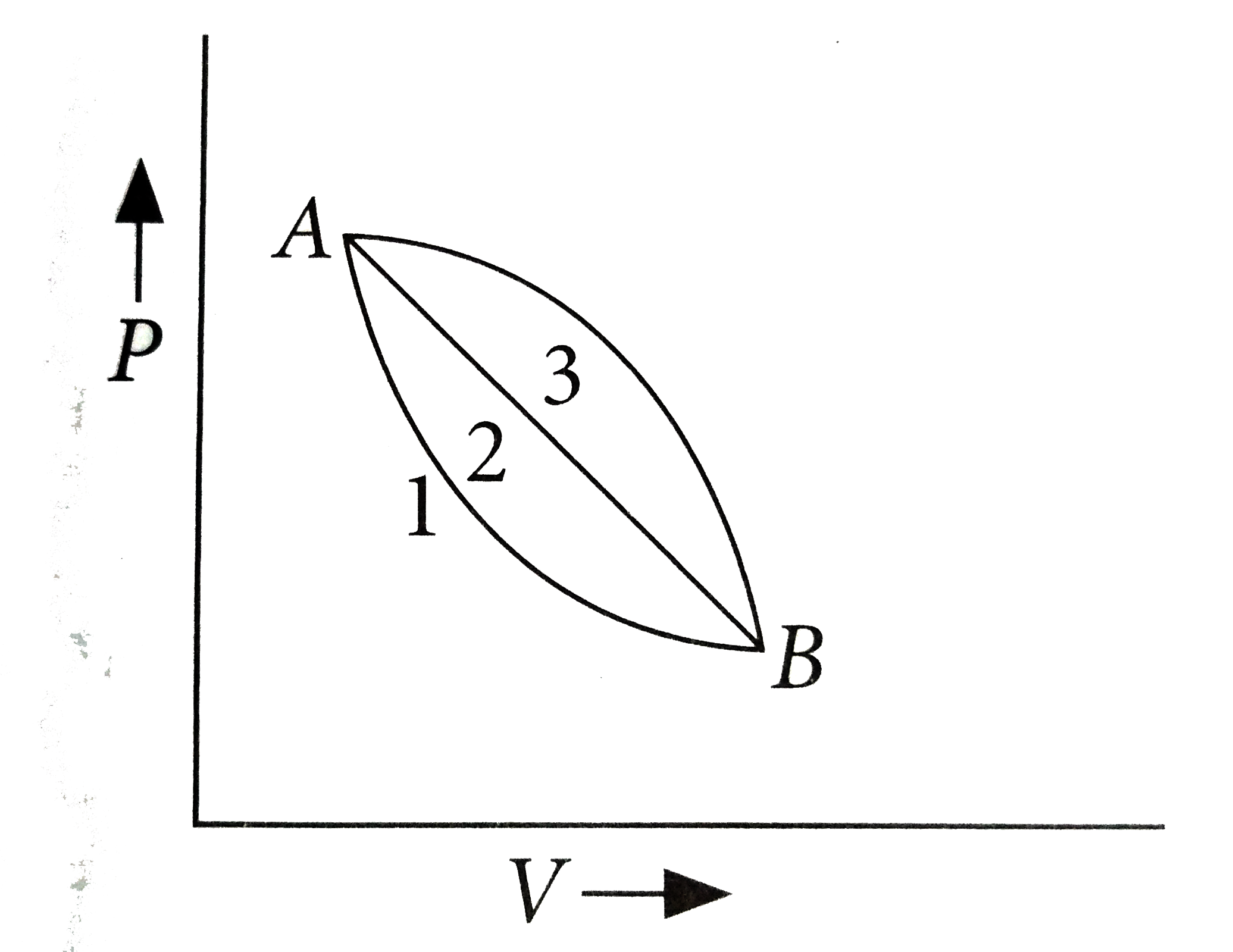
If w1,w2andw3 respectively, be the work done by the gas along three paths, then
View SolutionA givne mass of a gas expands from a state A to the state B by three paths 1, 2 and 3 as shown in T-V indicator diagram. If W_(1),W_(2) and W_(3) respectively be the work done by the gas along the three paths, then
View SolutionA given mass of gas expands from the state A to state B by three paths 1, 2 and 3 as shown in the figure. If w_1, w_2 and w_3 respectively be the work done by the gas along three paths then
View SolutionA given mass of gas expands from state A to state b by three paths 1,2 , and 3 as shown in the figure below. If w_(1),w_(2) and w_(3) , respectively, be the work done by the gas along three paths, then
View SolutionA given mass of a gas expands from the state A to the state B by three paths 1,2 and 3 as shown in the figure, If W1,W2 and W3 respectively be the work done by the gas along the three paths then
View Solution

