A piston cylinder device initially contains 0.2m3 neon (assume ideal ) at 200kPa inside at T1∘C. A valve is now opened and neon is allowed to escape until the volume reduces to half the initial volume. At the same time heat transfer with outside at T2∘C ensures a constant temperature inside. Select correct statement(s) for given process
 Solution in Telugu
Solution in Telugu
ΔU must be zero
ΔU can not be zero
q may be +ve
q may be -ve
Step by step video & image solution for A piston cylinder device initially contains 0.2m^(3) neon (assume ideal ) at 200kPa inside at T_(1) ""^(@)C. A valve is now opened and neon is allowed to escape until the volume reduces to half the initial volume. At the same time heat transfer with outside at T_(2) ""^(@)C ensures a constant temperature inside. Select correct statement(s) for given process by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
A monoatomic ideal gas, initially at temperature T_(1) is enclosed in a cylinder fitted with a frictionless piston. The gas is allowed to expand adiabatically to a temperature T_(2) by releasing the piston suddenly. If L_(1) and L_(2) are the lengths of the gas column. before and after expansion respectively, then (T_(1))/(T_(2)) is given by
View SolutionA piston cylinder device initially contains 0.2m3 neon (assume ideal ) at 200kPa inside at T1∘C. A valve is now opened and neon is allowed to escape until the volume reduces to half the initial volume. At the same time heat transfer with outside at T2∘C ensures a constant temperature inside. Select correct statement(s) for given process

View SolutionInitial temperature of an ideal gas is 75∘C. At what temperature, the sample of neon gas would be heated to double its pressure, if the initial volume of gas is reduced by 15%?
View SolutionA2 litre vessel is filled with air at 50∘C and pressure of 3 atm. The temperature is now raised to 200∘C A valve is now opened so that the pressure inside drops to one atm What fraction of the total number of moles, inside escaped on openig the valve ? Assume no change in the volume of the container .
View SolutionAt what temperature, the sample of neon gas would be heated to double of its pressure, if the initial volume of gas is reduced by 15% at 75∘C ?
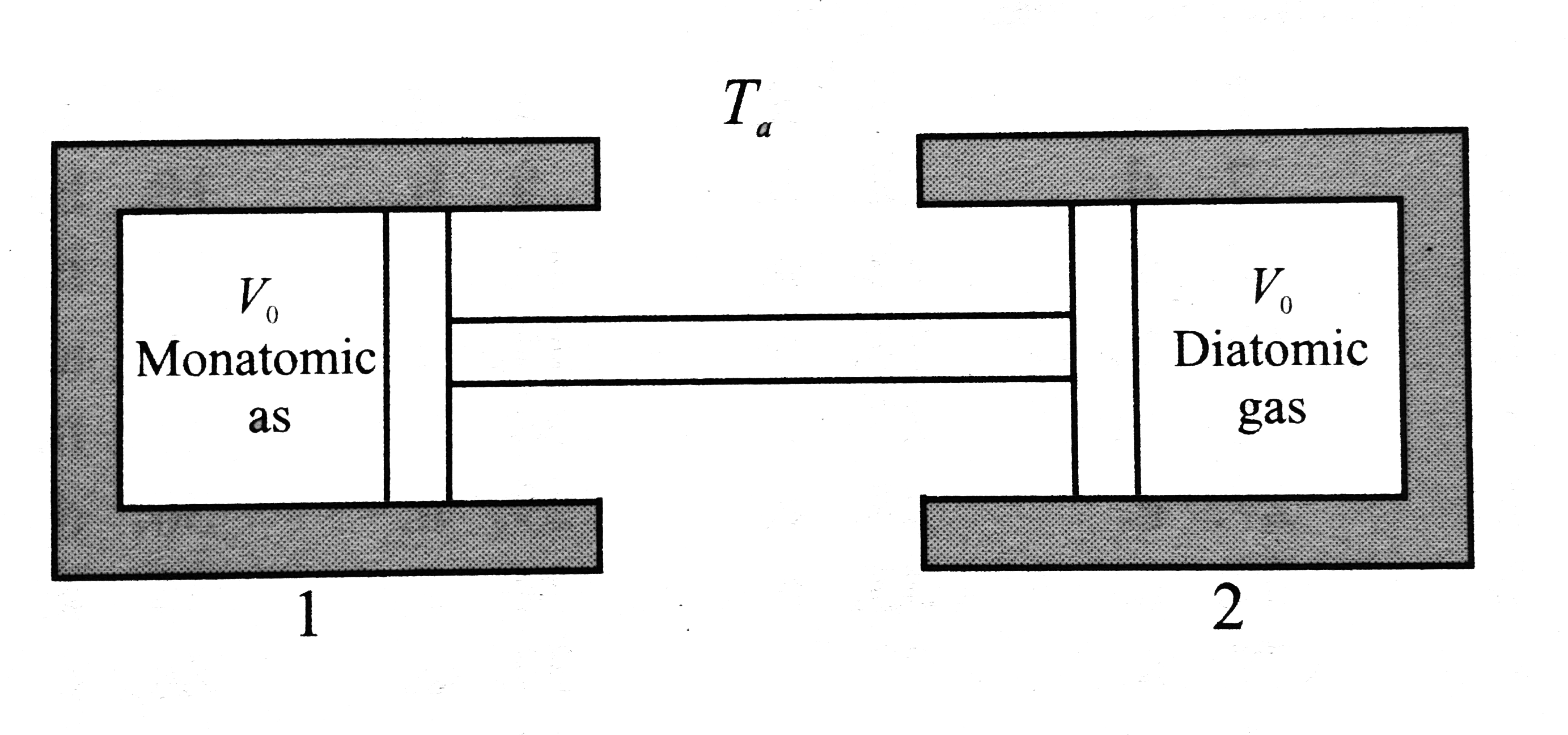
View SolutionThe two conducting cyliner-piston systems shows below are linked. Cyliner 1 is filled with a certain molar quantity of a monatomic ideal gas, and cylinder 2 is filled with an equal molar quantity of a diatomic ideal gas. The entire apparatus is situated inside an oven whose temperature is Ta=27∘C. The cylinder volumes have the same initial value V0=100. When the oven temperature is slowly raised to Tb=127∘C. What is the volume change ΔV (in cc) of cylinder 1 ?
View SolutionInitial temperature of an ideal gas is 75^(@)C. At what temperature, the sample of neon gas would be heated to double its pressure, if the initial volume of gas is reduced by 15%?
View SolutionThe temperature inside a refrigeratot is t_(2)^@C temperature is 't_(2)^@C . The amount of heat delivered to for each joule of electrical energy consumed ideally.
View Solution

