The nucleus 6429Cu accepts an orbital electron to yield
6528Ni
6430Zn
6428Ni
6530Zn
The correct Answer is:C
6429Cu+e− (K-electron capture) →6428Ni
Topper's Solved these Questions
- RADIOACTIVITY AND NUCLEAR CHEMISTRY Book:MTG-WBJEEChapter:RADIOACTIVITY AND NUCLEAR CHEMISTRY Exercise:WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 2 : SINGLE OPTION CORRECT TYPE)
Explore 1 Video
- RADIOACTIVITY AND NUCLEAR CHEMISTRY Book:MTG-WBJEEChapter:RADIOACTIVITY AND NUCLEAR CHEMISTRY Exercise:WB JEE WORKOUT (CATEGORY 3 : ONE OR MORE THAN ONE CORRECT TYPE)
Explore 10 Videos
- PRINCIPLES OF QUALITATIVE ANALYSISBook:MTG-WBJEEChapter:PRINCIPLES OF QUALITATIVE ANALYSISExercise:WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 2 : Single Option Correct Type)
Explore 2 Videos
- REDOX EQUILIBRIABook:MTG-WBJEEChapter:REDOX EQUILIBRIAExercise:WB JEE PREVIOUS YEARS QUESTIONS
Explore 5 Videos
Similar Questions
A piece of wood from an aechaeological sample has 5.0 counts min−1 per gram of C - 14, while a fresh sample of wood has a count of 15.0min−1. If half-life of C-14 is 5770 years, the age of the archaeological sample is
View SolutionThe number of αandβ emitted in the nuclear reaction 23892U→21482Pb is
View SolutionCompound X is tested and the results are shown in the table :

Which ions are present in compound X?
View SolutionThe two half cell reactions of an electrochemical cell is given as
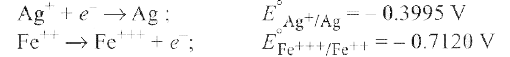
the value of cell EMF will be
View SolutionElectronic configurations of Cu(Z=29) is
View Solution.6429 Cu can decay by β− or β+ emmision, or electron capture. It is known that .6429 Cu has a half life of 12.8 hrs with 40% probability of β− decay 20% probability of β+ decay and 40% probability of electron capture. The mass of .6429 Cu is 63.92977aμwhile._(30)^(64) Zn is 63.92914 amuand._(28)^(64) Ni is 63.92796 amu.
What is the half life for electron capture?
View Solution._(29)^(64) Cu can decay by beta^(-) or beta^(+) emmision, or electron capture. It is known that ._(29)^(64) Cu has a half life of 12.8 hrs with 40% probability of beta^(-) decay 20% probability of beta^(+) decay and 40% probability of electron capture. The mass of ._(29)^(64) Cu is 63.92977amu whil e ._(30)^(64) Zn is 63.92914 amu and ._(28)^(64) Ni is 63.92796 amu . What is the Q value of beta^(-) decay?
View Solution.6429 Cu can decay by β− or β+ emmision, or electron capture. It is known that .6429 Cu has a half life of 12.8 hrs with 40% probability of β− decay 20% probability of β+ decay and 40% probability of electron capture. The mass of .6429 Cu is 63.92977aμwhile._(30)^(64) Zn is 63.92914 amuand._(28)^(64) Ni is 63.92796 amu.<br>If∈itiallytherewas10^(22)a→msof._(29)^(64)Cu,wˆisthe∈itialrateatwhiche≠rgyisbe∈g∏uceddue→beta^(+) decay ?
View SolutionThe valence electrons of ._(29)Cu lie in the
View Solutionthe radius of ""_(29)Cu^(64) nucleus in fermi is (given ,r_(0) 1.2xx10^(-15) M)
View SolutionWrite the electronic configuartion of Cu+ (At. No. of Cu= 29)
View Solutionइन के इलेक्ट्रॉनिक विन्यास लिखें।
Cu(29)
View SolutionCu (29) का शुद्ध इलेक्ट्रॉनिक विन्यास है
View SolutionThe nucleus 6429Cu accepts an orbital electron to yield-
View SolutionThe radius of ""_(29) Cu^(64) nucleus in Fermi is (given R_(0) = 1.2 xx 10^(-15) m )
View Solution

