In the anion RCOO−, the two carbon -oxygen bonds are found to be of equal length. What is the reason for it?
The anion is obtained by removal of a proton from the acid molecule.
Electronic orbitals of carbon atom are hybridised.
The C=O bond is weaker than the C-O bond.
The anion RCOO− has two resonating structures.
The correct Answer is:D
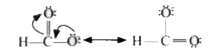
The anion HCOO− has two equivalent resonating structures :
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:MTG-WBJEEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:WB JEE WORKOUT (CATEGORY 2: SINGLE OPTION CORRECT TYPE)
Explore 15 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:MTG-WBJEEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:WB JEE WORKOUT(CATEGORY 3: ONE OR MORE THAN ONE OPTION CORRECT TYPE)
Explore 15 Videos
- ALCOHOLS AND ETHERSBook:MTG-WBJEEChapter:ALCOHOLS AND ETHERSExercise:WB JEE Previous years questions (CATEGORY 3: One or More than One Option Correct Type (2 Marks))
Explore 1 Video
- ALIPHATIC AMINESBook:MTG-WBJEEChapter:ALIPHATIC AMINESExercise:WB JEE Previous Years Questions ( CATEGORY 3 : One or More than One Option Corred Type (2 Marks) )
Explore 1 Video
Similar Questions
Knowledge Check
In the anion HCOO− , the two carbon-oxygen bonds are found to be of equal length. What is the reason for it ?
AThe C=O bond is weaker than the C−O bond.BThe anion HCOO− has two resonating structures.CThe electronic orbitals of carbon atom are hybridized.DThe anion is obtained by removal of proton from the acid molecule.In the anion HCOO− the two carbon- oxygen bonds are found to be of equal length. What is the reason for it :
AElectronic orbitals of carbon atom are hybridized.BThe C=O bond is weaker than the C−O bond.CThe anion HCOO− has two resonating structures.DThe anion is obtained by removal of a proton from the acid molecule.In the anion HCOO− the two carbon-oxygen bonds are founds to be of equal length. What is the reason for it?
AThe anion HCOO− has two equivalent resonating structuresBThe anion is obtained by removal of a proton from the acid moleculeCElectronic orbitals of carbon atom are hybridisedDThe C=O bond is weaker than the C−O bond
In the anion HCOO−, the two carbon-oxygen bonds are found to be equal length. What is the reason for it ?
View SolutionIn the anion HCOO−, the two carbon-oxygen bonds are found to be equal length. What is the reason for it?
View SolutionIn the HCOO− the two carbon-oxygen bonds are found to be of equal length. What is the reason for it ?
View SolutionIn an anion HCOO− the two carbon-oxygen bonds are found to be equal length. What is the reason for it?
View SolutionIn the anion HCOO− , the carbon-oxygen bonds are found to be of equal length. This is due to :
View Solution

