Read the pasage given below as well as the adjoining energy level diagram and then answer the questions given after the passage .
As per Bohr's theory og hydrogen atom the energy of an atom in a state corresponding to principal quantum number n is given as :
En=−13.6n2eV
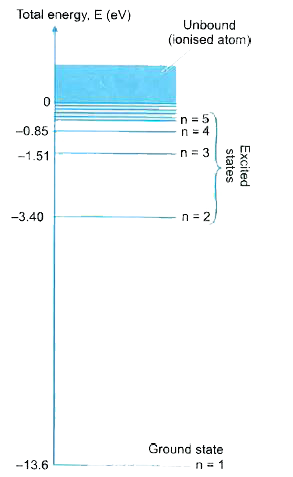
The energy of an atom is the least when its electron is revolving in an orbit closets to the nucleus i.e., the one for which n =1. This state is called the ground state. When a hydrogen atom receives energy by processes such as electron collision , the atom may acquire sufficient energy to raise the electron to higher energy states and the atom is then said to be in an excited state . From these excited states the electron can then fall back to a state of lower energy , emitting a photon in the process.
The energy level diagram for the stationary states of a hydrogen atom , compouted from Bohr's relation for energy , is given in Fig.12.02. The principal quantum number n labels the stationary states in the ascending order of energy . Obviously , the highest energy corresponds to n=∞ and has an energy of 0 eV. This is the energy of the atom when the electron is completely removed (r=∞) from the nucleus and is at rest.
Now answer the follwoing questions:
Define ionisation energy of hydrogen atom and give its value.
Minimum amount of energy required to free electron of hydrogen atom from its ground state is called its ionisation energy . Its value is + 13.6 eV.
Similar Questions
Read the pasage given below as well as the adjoining energy level diagram and then answer the questions given after the passage .
As per Bohr's theory og hydrogen atom the energy of an atom in a state corresponding to principal quantum number n is given as :
En=−13.6n2eV
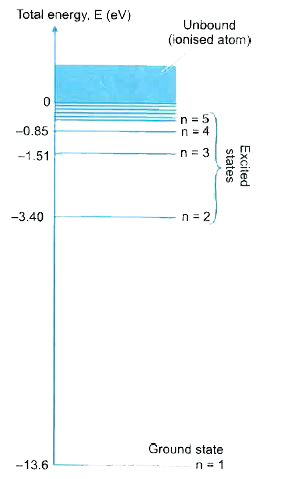
The energy of an atom is the least when its electron is revolving in an orbit closets to the nucleus i.e., the one for which n =1. This state is called the ground state. When a hydrogen atom receives energy by processes such as electron collision , the atom may acquire sufficient energy to raise the electron to higher energy states and the atom is then said to be in an excited state . From these excited states the electron can then fall back to a state of lower energy , emitting a photon in the process.
The energy level diagram for the stationary states of a hydrogen atom , compouted from Bohr's relation for energy , is given in Fig.12.02. The principal quantum number n labels the stationary states in the ascending order of energy . Obviously , the highest energy corresponds to n=∞ and has an energy of 0 eV. This is the energy of the atom when the electron is completely removed (r=∞) from the nucleus and is at rest.
Now answer the follwoing questions:
When does a hydrogen atom emit a photon ?
View SolutionRead the pasage given below as well as the adjoining energy level diagram and then answer the questions given after the passage .
As per Bohr's theory og hydrogen atom the energy of an atom in a state corresponding to principal quantum number n is given as :
En=−13.6n2eV
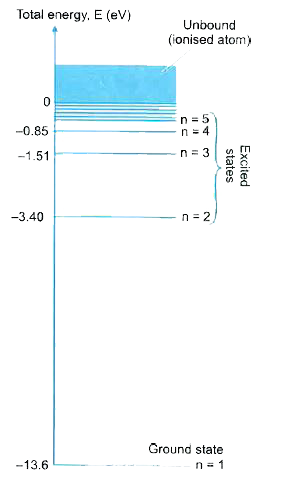
The energy of an atom is the least when its electron is revolving in an orbit closets to the nucleus i.e., the one for which n =1. This state is called the ground state. When a hydrogen atom receives energy by processes such as electron collision , the atom may acquire sufficient energy to raise the electron to higher energy states and the atom is then said to be in an excited state . From these excited states the electron can then fall back to a state of lower energy , emitting a photon in the process.
The energy level diagram for the stationary states of a hydrogen atom , compouted from Bohr's relation for energy , is given in Fig.12.02. The principal quantum number n labels the stationary states in the ascending order of energy . Obviously , the highest energy corresponds to n=∞ and has an energy of 0 eV. This is the energy of the atom when the electron is completely removed (r=∞) from the nucleus and is at rest.
Now answer the follwoing questions:
Which atomic transition correspond to spectral lines of hydrogen in visible light ?
View SolutionRead the pasage given below as well as the adjoining energy level diagram and then answer the questions given after the passage . As per Bohr's theory og hydrogen atom the energy of an atom in a state corresponding to principal quantum number n is given as : E_(n) = - (13.6)/(n^(2)) eV The energy of an atom is the least when its electron is revolving in an orbit closets to the nucleus i.e., the one for which n =1. This state is called the ground state. When a hydrogen atom receives energy by processes such as electron collision , the atom may acquire sufficient energy to raise the electron to higher energy states and the atom is then said to be in an excited state . From these excited states the electron can then fall back to a state of lower energy , emitting a photon in the process. The energy level diagram for the stationary states of a hydrogen atom , compouted from Bohr's relation for energy , is given in Fig.12.02. The principal quantum number n labels the stationary states in the ascending order of energy . Obviously , the highest energy corresponds to n = oo and has an energy of 0 eV. This is the energy of the atom when the electron is completely removed (r= oo) from the nucleus and is at rest. Now answer the follwoing questions: Name the spectral series of hydrogen atom observed in visible light region.
View SolutionRead the pasage given below as well as the adjoining energy level diagram and then answer the questions given after the passage . As per Bohr's theory og hydrogen atom the energy of an atom in a state corresponding to principal quantum number n is given as : E_(n) = - (13.6)/(n^(2)) eV The energy of an atom is the least when its electron is revolving in an orbit closets to the nucleus i.e., the one for which n =1. This state is called the ground state. When a hydrogen atom receives energy by processes such as electron collision , the atom may acquire sufficient energy to raise the electron to higher energy states and the atom is then said to be in an excited state . From these excited states the electron can then fall back to a state of lower energy , emitting a photon in the process. The energy level diagram for the stationary states of a hydrogen atom , compouted from Bohr's relation for energy , is given in Fig.12.02. The principal quantum number n labels the stationary states in the ascending order of energy . Obviously , the highest energy corresponds to n = oo and has an energy of 0 eV. This is the energy of the atom when the electron is completely removed (r= oo) from the nucleus and is at rest. Now answer the follwoing questions: Calculate the wavelenght of first spectral lines of series mentioned in part (g) of question.
View SolutionRead the pasage given below as well as the adjoining energy level diagram and then answer the questions given after the passage .
As per Bohr's theory og hydrogen atom the energy of an atom in a state corresponding to principal quantum number n is given as :
En=−13.6n2eV
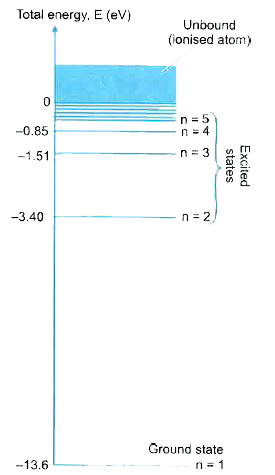
The energy of an atom is the least when its electron is revolving in an orbit closets to the nucleus i.e., the one for which n =1. This state is called the ground state. When a hydrogen atom receives energy by processes such as electron collision , the atom may acquire sufficient energy to raise the electron to higher energy states and the atom is then said to be in an excited state . From these excited states the electron can then fall back to a state of lower energy , emitting a photon in the process.
The energy level diagram for the stationary states of a hydrogen atom , compouted from Bohr's relation for energy , is given in Fig.12.02. The principal quantum number n labels the stationary states in the ascending order of energy . Obviously , the highest energy corresponds to n=∞ and has an energy of 0 eV. This is the energy of the atom when the electron is completely removed (r=∞) from the nucleus and is at rest.
Now answer the follwoing questions:
Find a relation between wavelengths of first line and series limit of hydrogen spectrum in vissible light.
View SolutionRead the pasage given below as well as the adjoining energy level diagram and then answer the questions given after the passage . As per Bohr's theory og hydrogen atom the energy of an atom in a state corresponding to principal quantum number n is given as : E_(n) = - (13.6)/(n^(2)) eV The energy of an atom is the least when its electron is revolving in an orbit closets to the nucleus i.e., the one for which n =1. This state is called the ground state. When a hydrogen atom receives energy by processes such as electron collision , the atom may acquire sufficient energy to raise the electron to higher energy states and the atom is then said to be in an excited state . From these excited states the electron can then fall back to a state of lower energy , emitting a photon in the process. The energy level diagram for the stationary states of a hydrogen atom , compouted from Bohr's relation for energy , is given in Fig.12.02. The principal quantum number n labels the stationary states in the ascending order of energy . Obviously , the highest energy corresponds to n = oo and has an energy of 0 eV. This is the energy of the atom when the electron is completely removed (r= oo) from the nucleus and is at rest. Now answer the follwoing questions: Write the formula for spectral lines the of hydrogen atom in visible light in terms of Rydberg's constant.
View SolutionThe energy of a hydrogen atom in its ground state is -13.6 eV . The energy of the level corresponding to the quantum number n=5 is
View Solution

