What type of defect can arise when a solid is heated ? Which physical property is affected by it and in what way ?
On heating, some atoms or ions leave the lattice positions because of increased kinetic energy. Thus, some vacancies are created at some lattice sites. This results in decrease of density.
Topper's Solved these Questions
- SOLID STATEBOOK - U-LIKE SERIESCHAPTER - SOLID STATEEXERCISE - NCERT TEXTBOOK EXERCISES
26 Videos
- SOLID STATEBOOK - U-LIKE SERIESCHAPTER - SOLID STATEEXERCISE - CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS
14 Videos
- SAMPLE QUESTIONS PAPER BOOK - U-LIKE SERIESCHAPTER - SAMPLE QUESTIONS PAPER EXERCISE - SECTION D
6 Videos
- SOLUTIONBOOK - U-LIKE SERIESCHAPTER - SOLUTIONEXERCISE - SELF ASSESSMENT TEST (SECTION D)
2 Videos
Similar Questions
Knowledge Check
What is the effect on chemical properties and physical properties of water when temperature is changed ?
AChemical properties of water remain same but the physical state changes with change in temperature.BChemical properties of water change with change in temperature but physical properties remain same.CThere is no effect on chemical or physical properties of water when temperature is changed.DBoth chemical and physical properties of water change with change in temperature.What is the effect on chemical properties and physical properties of water when temperature is changed ?
AChemical properties of water remain same but the physical state changes with change in temperature.BChemical properties of water change with change in temperature but physical properties remain same.CThere is no effect on chemical or physical properties of water when temperature is changed.DBoth chemical and physical properties of water change with change in temperature.Solid which do not show the same physical properties in different directions are called:
APseudo solidsBIsotropic solidsCPolymorphic solidsDAnisotropic solids
When a solid is heated, what happens to its molecules?
View SolutionWhat is Schottky defect in a solid ?
View SolutionExamine the illustration of a portion of the defective crystal given below and answer the following questions :
(i) What are these type of vacancy defects called ?
(ii) How is the density of a crystal affected by these defects ?
(iii) Name one ionic compound which can show this type of defect in the crystalline state.
(iv) How is the stoichiometry of the compound affected ?

View SolutionExamine the illustration of a portion of the deffective crystal and answer the following question :
(a) What are these type of vacancy defects called ?
(b) How is the density of a crystal affected by these defects ?
(c) Name one ionic compound which can show this type of defect in the crystalline state.
(d) How is the stoichiometry of the compound affected ?
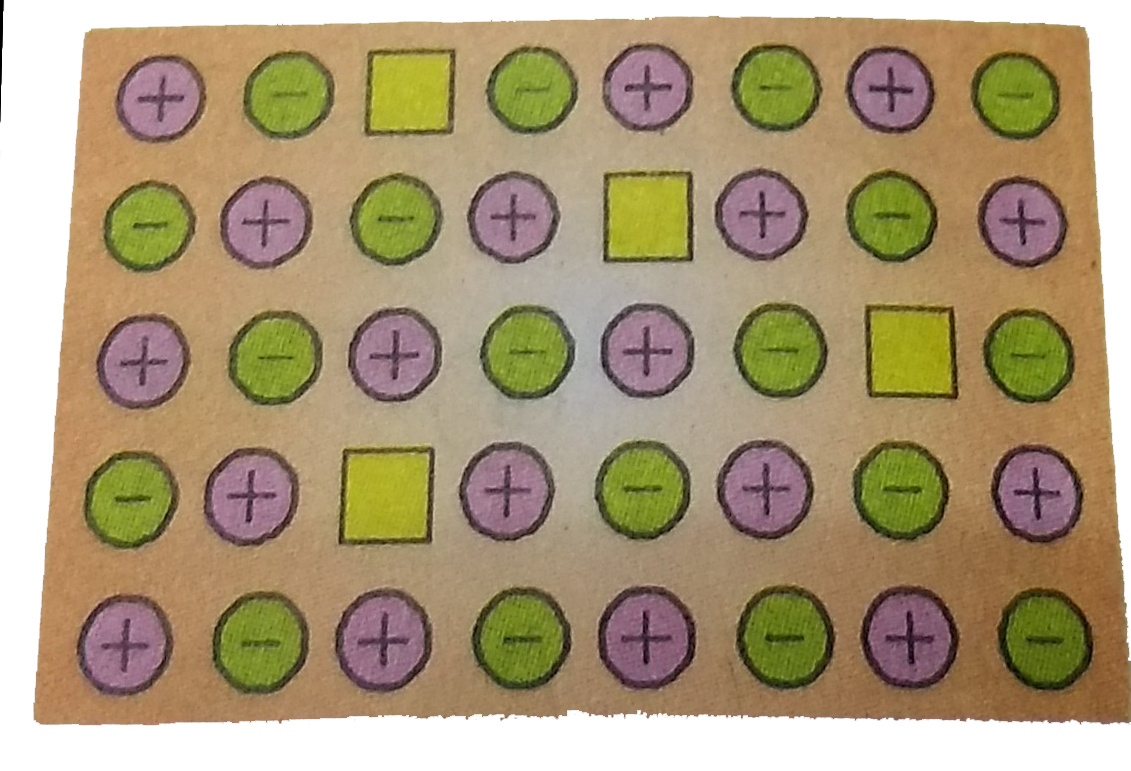
View SolutionExamine the illustration of a portion of the deffective crystal and answer the following question :
(a) What are these type of vacancy defects called ?
(b) How is the density of a crystal affected by these defects ?
(c) Name one ionic compound which can show this type of defect in the crystalline state.
(d) How is the stoichiometry of the compound affected ?

View Solution

