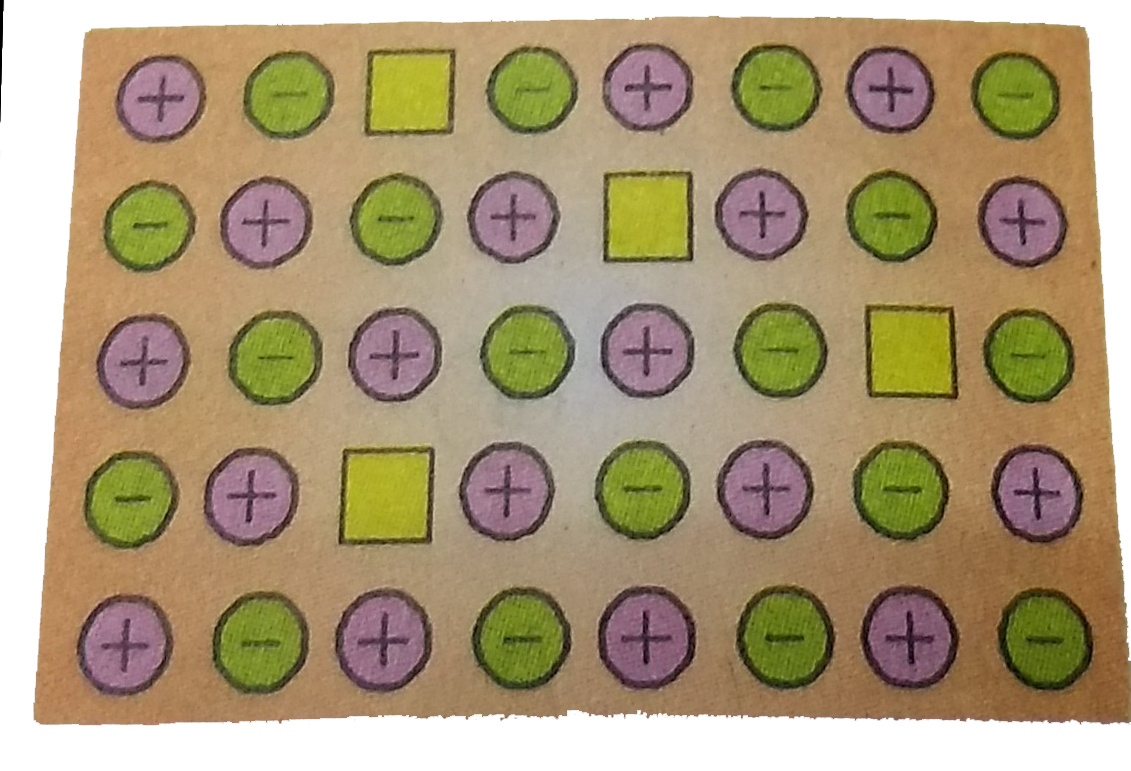
Examine the given defective crystal and
A+B−A+B−A+B−0B−A+B−A+B−A+0A+B−A+B−A+B−
Answer the following questions :
(i) What type of stoichiometirc defect is shown by the crystal ?
(ii) How is the density of the crystal affected by this defect ?
(iii) What type of ionic substances show such defect ? Solution in Kannada
(i) Schottky defect.
(ii) Decreases.
(iii) Alkali metal halides/Ionic substances having almost similar size of cations and anions (NaCl/KCl).
Similar Questions
Examine the given defective crystal

Answer the following questions.
What type of stoichiometric defect is shown by the crystal?
View SolutionExamine the given defective crystal Answer the following questions. How is the density of the crystal affected by this defect?
View SolutionExamine the illustration of a portion of the deffective crystal and answer the following question :
(a) What are these type of vacancy defects called ?
(b) How is the density of a crystal affected by these defects ?
(c) Name one ionic compound which can show this type of defect in the crystalline state.
(d) How is the stoichiometry of the compound affected ?
View SolutionExamine the given defective crystal?

Answer the following questions:
What type of stoichiometric defect is shown by the crystal?
View SolutionExamine the given defective crystal?

Answer the following questions:
How is the density of the crystal affected by this defect?
View SolutionExamine the given defective crystal

Answer the following questions:
What type of stoichiometric defect is shown by the crystal?
View SolutionExamine the given defective crystal Answer the following questions: What type of ionic substances show such defect?
View SolutionExamine the illustration of a portion of the deffective crystal and answer the following question :
(a) What are these type of vacancy defects called ?
(b) How is the density of a crystal affected by these defects ?
(c) Name one ionic compound which can show this type of defect in the crystalline state.
(d) How is the stoichiometry of the compound affected ?

View Solution

