Explain why a solution of chloroform and acetone shows negative deviation from Raoult's law. Solution in Kannada
Video Solution
Text Solution
Solution of chloroform and acetone shows negative deviation from Raoult. s law : Negative deviation is shown when vapour pressure of resulting solution is low and its boiling point is high. Chloroform and acetone on mixing form
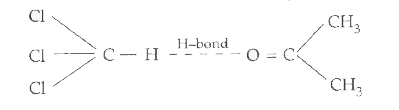
(H-Bond in acetone and chloroform molecules) Since new attractive forces which come into existence in these solutions are relatively stronger, therefore these solutions are accompanied by evolution of heat (ΔHmix=- ve).
Also because of stronger forces of attraction among molecules in solution, the molecules come closer and hence volume of solution is less than the total
(ΔVmax−ve) .
|
Similar Questions
Statement-1: Acetone + Carbon disulphide solution shows positive deviation from Raoult's law
Statement-2 : Acetone + Aniline solution shows positive deviation from Raoult's law
View SolutionMixture of chloroform and acetone shown a negative deviation from Raoult's Law. Explain.
View SolutionThe solution which show positive or negative deviation from Raoult's law are called:
View SolutionExplain why a solution of chloroform and aceton shows negative deviation from Raoult's law?
View SolutionThe liquid pair of acetone-chloroform shows a positive deviation form Raoult's law.
View SolutionMixture of chloroform and acetone shows a negative deviation from Raoult's law. Explain.
View SolutionSolutions showing negative deviations from Raoult's law
View Solution

