State reason for the following
The molecular shape of Ni(CO)4 is not the same as that of [Ni(CN)4]2− .Solution in Kannada
Video Solution
Text Solution
Electronic configuration of :
Ni:[Ar]4s2,3d8
Ni (O) :[Ar]4s0,3d10
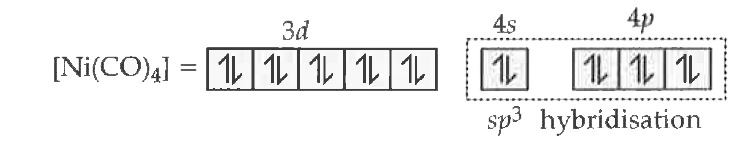
[Ni(CO)4 has sp3 hybridisation and has tetrahedral shape.
[Ni(CN)4)−2
Ni2+,4s03d8
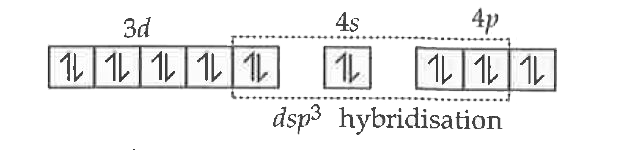
|
Similar Questions
Among [Ni(CN)4]2−,[NiCl4]2− and [Ni(CO)4]:
View SolutionAmong Ni(CO)_4Ni(CN)_4^(2-) and Ni(CN)_4^(2-) :
View SolutionExplain why [Ni(CN)4]2− and [Ni(CO)4] having same magnetic property through they differ in geometry?
View SolutionAmong [Ni (CO)_4], [Ni(CN)_4]^(2-), [NiCl_4]^(2-) species, the hybridisation states at the Ni atom are, respectively (Atomic number.of Ni=28)
View SolutionAmongst Ni(CO)4,[Ni(CN)4]2− and [NiCl4]2−
View SolutionAmongst Ni(CO)4,[Ni(CN)4]2− and [NiCl4]2−
View SolutionState a reason for the following situation : The molecular shape of Ni(CO)4 is not the same as that of [Ni(CN)4]2−?
View Solution[Ni(CN)4]2− is diamagnetic and has ............. shape.
View Solution
