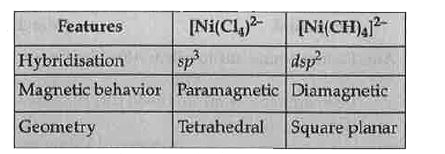
Give differences between [NiCl4]2−and[NI(CN)4]2− with respect to type hybridization, magnetic behaviour and geometry.Solution in Kannada
Video Solution
Text Solution
|
Similar Questions
Ni(CO)4,[Ni(CN)4]2− तथा [NiCl4]2−में
View SolutionAmong [Ni(CO)4],[Ni(CN)4]−2 and [NiCl4]−2 species the hybridisation state of the Ni atom are respectively?
View SolutionAmong [Ni(CO)4],[Ni(CN)4]−2 and [NiCl4]−2 species the hybridisation state of the Ni atom are respectively?
View SolutionExplain, why [Ni(CN)_4]^(2-) and [NiCl_4]^(2-) have different geometry.
View SolutionDiscuss the shape and magnetic property of [Ni(CN)_4]^(2-) and [NiCl_4]^(2-) .
View SolutionAmongst Ni(CO)4,[Ni(CN)4]2− and [NiCl4]2−
View SolutionAccount for the different magnetic behaviour of [Ni(CN)4]2−and[NiCl4]2− .
View SolutionAmongst Ni(CO)4,[Ni(CN)4]2− and [NiCl4]2−
View Solution