With the help of valence bond theory account for the geometry and magnetic property of (Co(NH3)6)3+.Solution in Kannada
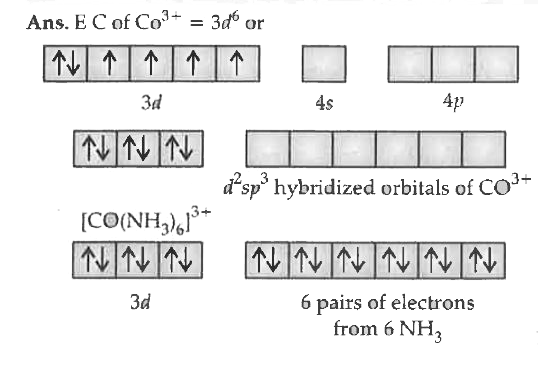
Geometry : Octahedral
Magnetic property : Diamagnetic
Similar Questions
Using valence bond theory (VBT), account for the geometry, type of hybridization and magnetic property of [NiCl4]2−.
View SolutionWith the help of valence bond theory account for the geometry and magnetic property of (Co(NH3)6)3+.
View SolutionUsing valence bond theory explain geometry, hybridisation and magnetic property of [CoF_6]_3^- (Atomic number of Co = 27).
View SolutionUsing the valence bond theory predict the geometry and magnetic behaviour of [CoF6]3−.
[At. no. of Co = 27]
View SolutionUsing valence bond theory explain geometry, hybridisation and magnetic property of [CoF6]−3 (Atomic number of Co = 27).
View SolutionWith the help of valence bond theory explain hybridization, geometry and magnetic property of Nickel tetracarbonyl.
View SolutionOn the basis of VBT explain the hybridization, geometrical shape and magnetic property of [CoF6]3−, hexafluorido cobaltate (III) ion.
View SolutionUsing valence bond theory explain geometry, hybridisation and magnetic property of [CoF6]−3 (Atomic number of Co = 27).
View Solution
