With the help of VBT explain the hybridisation in tetracarbonyl nickel and sketch the shape of the complex.Solution in Kannada
Video Solution
Text Solution
Electronic configuration of Ni:[Ar]3d84s2 pairing of electrons under the influence of CO Ligands.
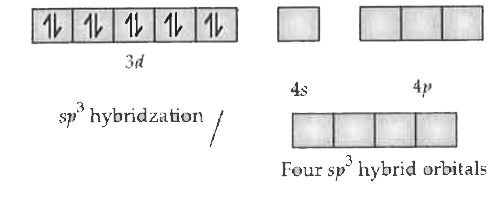
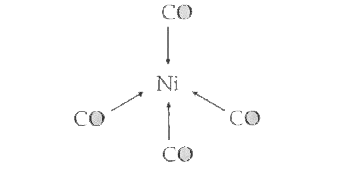
|
Similar Questions
With the help of Valence Bond Theory (VBT) explain hybridisation, geometry and magnetic property of [CoF_6]^2 heat fluoridocobaltate (III) ion.
View SolutionExplain the hybridisation, geometry and magnetic property of [Ni(Cl)4]2−.
View SolutionThe complex [Ni(CN)_(4)]^(2-) is diamagnetic and it involves the following hybridisation of nickel :
View SolutionWith the help of valence bond theory explain hybridization, geometry and magnetic property of Nickel tetracarbonyl.
View SolutionExplain the shape and the bond angle in BeCl2in terms of VBT
View SolutionThe hybridisation of nickel in tetracarbonyl nickel is
View SolutionVBT |Hybridisation
View Solution
