Explain the mechanism of addition of HCN to a carbonyl group in presence of a base.Solution in Kannada
Video Solution
Text Solution
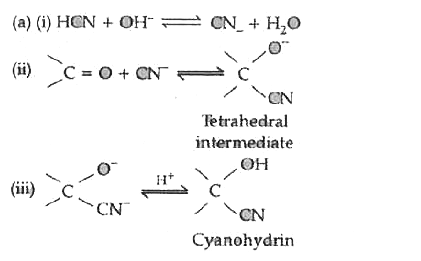
The mechanism of addition of HCN to carbonyl group takes places according to the following steps.
Step - (1) : Addition of nucleophilic (CN−) to carbonyl carbon.
The nucleophilic attacks the sp2 hybridised carbonyl carbon atom forming tetrahedral alkoxide intermediate product.

Step - (2) : Addition of proton of alkoxide ion.
Alkoxide ion intermediate product picks up a proton from the reaction medium to form the electrically neutral final product.
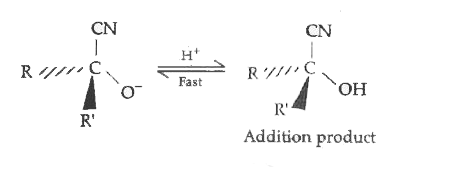
|
Similar Questions
Give the mechanism of cyanohydrin formation when carbonyl compounds reaction with HCN in presence of alkali.
View SolutionExplain the mechanism of additon of HCN to a carbonyl group presence of a base.
View SolutionExplain the addition of mechanism of HCN to aldehyde or ketones.
View SolutionExplain the mechanism of addition of HCN to acetaldehyde.
View SolutionExplain the mechanism of addition of HCN to a carbonyl group in presence of a base.
View SolutionThe addition of HCN to carbonyl compounds is an example of .............. reaction.
View SolutionThe addition of HCN to carbonyl compounds is an example of
View SolutionExplain the mechanism of nucleophilic addition to a carbonyl group and give one example of such addition reactions.
View Solution

